Insight into the role of phosphatidylserine in complement-mediated synapse loss in Alzheimer's disease
- PMID: 33718936
- PMCID: PMC7946395
- DOI: 10.12703/r/10-19
Insight into the role of phosphatidylserine in complement-mediated synapse loss in Alzheimer's disease
Abstract
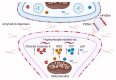
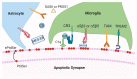
The innate immune system plays an integral role in the brain. Synaptic pruning, a fundamental process in developmental circuit refinement, is partially mediated by neuroimmune signalling at the synapse. In particular, microglia, the major tissue-resident macrophages of the brain, and the classical complement cascade, an innate immune pathway that aids in the clearance of unwanted material, have been implicated in mediating synapse elimination. Emerging data suggest that improper signalling of the innate immune pathway at the synapse leads to pathological synapse loss in age-related neurodegenerative diseases, including Alzheimer's disease. Now the key questions are whether synapses are targeted by complement and, if so, which synapses are vulnerable to elimination. Here, we review recent work implicating C1q, the initiator of the classical complement cascade, and surrounding glia as mediators of synapse loss. We examine how synapses could undergo apoptosis-like pathways in the Alzheimer brain, which may lead to the externalisation of phosphatidylserine on synapses. Finally, we discuss potential roles for microglia and astrocytes in this 'synaptic apoptosis'. Critical insight into neuroimmune regulatory pathways on synapses will be key to developing effective targets against pathological synapse loss in dementia.
Keywords: Alzheimer’s disease; MFG-E8; TAM; TREM2; astrocyte; caspase-3; classical complement cascade; microglia; mitochondrial dysfunction; phosphatidylserine; synapse loss; synaptosis.
Copyright: © 2021 Hong S et al.
Conflict of interest statement
The following patents have been granted or applied for: PCT/2015/010288, US14/988387 and EP14822330 (S.H.). All the other authors declare that they have no competing interests.Morgan Sheng is a member of the Board of Directors for Prevail Therapeutics, a Co-Founder and SAB member for RBNC, consults for Genentech Inc, and is an SAB member for Biogen Inc and Cerevel Therapeutics, LLC.No competing interests were disclosed.No competing interests were disclosed.
Figures
Similar articles
-
Microglial clearance of focal apoptotic synapses.Neurosci Lett. 2019 Aug 10;707:134317. doi: 10.1016/j.neulet.2019.134317. Epub 2019 Jun 5. Neurosci Lett. 2019. PMID: 31175934 Review.
-
Identification of Neuronal Pentraxins as Synaptic Binding Partners of C1q and the Involvement of NP1 in Synaptic Pruning in Adult Mice.Front Immunol. 2021 Feb 8;11:599771. doi: 10.3389/fimmu.2020.599771. eCollection 2020. Front Immunol. 2021. PMID: 33628204 Free PMC article.
-
Complement C1q-dependent excitatory and inhibitory synapse elimination by astrocytes and microglia in Alzheimer's disease mouse models.Nat Aging. 2022 Sep;2(9):837-850. doi: 10.1038/s43587-022-00281-1. Epub 2022 Sep 20. Nat Aging. 2022. PMID: 37118504 Free PMC article.
-
Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia.EMBO J. 2020 Aug 17;39(16):e105380. doi: 10.15252/embj.2020105380. Epub 2020 Jul 13. EMBO J. 2020. PMID: 32657463 Free PMC article.
-
Emerging evidence of context-dependent synapse elimination by phagocytes in the CNS.J Leukoc Biol. 2024 Sep 2;116(3):511-522. doi: 10.1093/jleuko/qiae098. J Leukoc Biol. 2024. PMID: 38700080 Review.
Cited by
-
An aging-sensitive compensatory secretory phospholipase that confers neuroprotection and cognitive resilience.bioRxiv [Preprint]. 2024 Sep 3:2024.07.26.605338. doi: 10.1101/2024.07.26.605338. bioRxiv. 2024. PMID: 39211220 Free PMC article. Preprint.
-
Neuroinflammation in Alzheimer disease.Nat Rev Immunol. 2025 May;25(5):321-352. doi: 10.1038/s41577-024-01104-7. Epub 2024 Dec 9. Nat Rev Immunol. 2025. PMID: 39653749 Review.
-
Microglia-synapse engulfment via PtdSer-TREM2 ameliorates neuronal hyperactivity in Alzheimer's disease models.EMBO J. 2023 Oct 4;42(19):e113246. doi: 10.15252/embj.2022113246. Epub 2023 Aug 14. EMBO J. 2023. PMID: 37575021 Free PMC article.
-
Assaying Microglia Functions In Vitro.Cells. 2022 Oct 28;11(21):3414. doi: 10.3390/cells11213414. Cells. 2022. PMID: 36359810 Free PMC article. Review.
-
Mass Spectrometry Chromatography-Based Metabolomics: The Effect of Long-Term Aerobic Exercise on Learning Ability and the Metabolism of Intestinal Contents in Mice with Alzheimer's Disease.Metabolites. 2023 Nov 14;13(11):1150. doi: 10.3390/metabo13111150. Metabolites. 2023. PMID: 37999246 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
