Anthracycline-Induced Cardiotoxicity: Molecular Insights Obtained from Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPSC-CMs)
- PMID: 33719006
- PMCID: PMC7956936
- DOI: 10.1208/s12248-021-00576-y
Anthracycline-Induced Cardiotoxicity: Molecular Insights Obtained from Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPSC-CMs)
Abstract
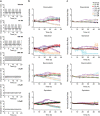
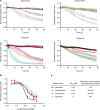
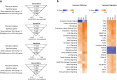
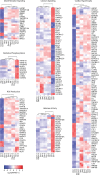
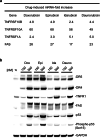
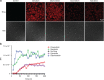
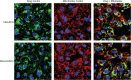
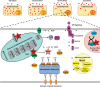
Anthracyclines are a class of chemotherapy drugs that are highly effective for the treatment of human cancers, but their clinical use is limited by associated dose-dependent cardiotoxicity. The precise mechanisms by which individual anthracycline induces cardiotoxicity are not fully understood. Human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) are emerging as a physiologically relevant model to assess drugs cardiotoxicity. Here, we describe an assay platform by coupling hiPSC-CMs and impedance measurement, which allows real-time monitoring of cardiomyocyte cellular index, beating amplitude, and beating rate. Using this approach, we have performed comparative studies on a panel of four anthracycline drugs (doxorubicin, epirubicin, idarubicin, and daunorubicin) which share a high degree of structural similarity but are associated with distinct cardiotoxicity profiles and maximum cumulative dose limits. Notably, results from our hiPSC-CMs impedance model (dose-dependent responses and EC50 values) agree well with the recommended clinical dose limits for these drugs. Using time-lapse imaging and RNAseq, we found that the differences in anthracycline cardiotoxicity are closely linked to extent of cardiomyocyte uptake and magnitude of activation/inhibition of several cellular pathways such as death receptor signaling, ROS production, and dysregulation of calcium signaling. The results provide molecular insights into anthracycline cardiac interactions and offer a novel assay system to more robustly assess potential cardiotoxicity during drug development.
Keywords: anthracycline; cardiotoxicity; cellular model; hiPSC-CMs.
Figures
References
-
- Venkatesh P, Kasi A. Anthracyclines. Treasure Island (FL): StatPearls; 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

