Effect of Professional CGM (pCGM) on Glucose Management in Type 2 Diabetes Patients in Primary Care
- PMID: 33719598
- PMCID: PMC8120045
- DOI: 10.1177/1932296821998724
Effect of Professional CGM (pCGM) on Glucose Management in Type 2 Diabetes Patients in Primary Care
Abstract
Background: Little data exists regarding the impact of continuous glucose monitoring (CGM) in the primary care management of type 2 diabetes (T2D). We initiated a quality improvement (QI) project in a large healthcare system to determine the effect of professional CGM (pCGM) on glucose management. We evaluated both an MD and RN/Certified Diabetes Care and Education Specialist (CDCES) Care Model.
Methods: Participants with T2D for >1 yr., A1C ≥7.0% to <11.0%, managed with any T2D regimen and willing to use pCGM were included. Baseline A1C was collected and participants wore a pCGM (Libre Pro) for up to 2 weeks, followed by a visit with an MD or RN/CDCES to review CGM data including Ambulatory Glucose Profile (AGP) Report. Shared-decision making was used to modify lifestyle and medications. Clinic follow-up in 3 to 6 months included an A1C and, in a subset, a repeat pCGM.
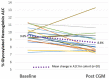
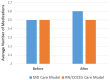
Results: Sixty-eight participants average age 61.6 years, average duration of T2D 15 years, mean A1C 8.8%, were identified. Pre to post pCGM lowered A1C from 8.8% ± 1.2% to 8.2% ± 1.3% (n=68, P=0.006). The time in range (TIR) and time in hyperglycemia improved along with more hypoglycemia in the subset of 37 participants who wore a second pCGM. Glycemic improvement was due to lifestyle counseling (68% of participants) and intensification of therapy (65% of participants), rather than addition of medications.
Conclusions: Using pCGM in primary care, with an MD or RN/CDCES Care Model, is effective at lowering A1C, increasing TIR and reducing time in hyperglycemia without necessarily requiring additional medications.
Keywords: glucose management; primary care; professional continuous glucose monitoring; quality improvement; type 2 diabetes.
Conflict of interest statement
RMB has received research support, consulted, or has been on a scientific advisory board for Abbott Diabetes Care, Ascensia, CeQur Corporation, DexCom, Hygieia, Insulet, Johnson & Johnson, Lilly, Medtronic, Novo Nordisk, Onduo, Roche, Sanofi and United Healthcare. His technology research is funded in part by NIH/NIDDK. RMB’s employer, non-profit HealthPartners Institute, contracts for his services and no personal income goes to RMB.
JLD reports no conflicts of interest.
MLJ has received research support from Abbott Diabetes Care, CeQur Corporation, DexCom, Hygieia, Insulet, JDRF, Lilly, Medtronic, NIH/NIDDK, Novo Nordisk, and Sanofi. MLJ’s employer, non-profit HealthPartners Institute, contracts for her services and no personal income goes to MLJ.
TWM is personally involved in a number of industry funded or sponsored research studies with Dexcom, Medtronic, Abbott Diabetes Care, Insulet, Novo-Nordisk, and Eli Lilly. His employer, non-profit HealthPartners Institute, contracts for his services and no personal income goes to TWM.
Figures


References
-
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853. - PubMed
-
- Edelman SV, Polonsky WH. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care. 2017;40(11):1425-1432. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

