Respiratory depression and analgesia by opioid drugs in freely behaving larval zebrafish
- PMID: 33720013
- PMCID: PMC8060028
- DOI: 10.7554/eLife.63407
Respiratory depression and analgesia by opioid drugs in freely behaving larval zebrafish
Abstract
An opioid epidemic is spreading in North America with millions of opioid overdoses annually. Opioid drugs, like fentanyl, target the mu opioid receptor system and induce potentially lethal respiratory depression. The challenge in opioid research is to find a safe pain therapy with analgesic properties but no respiratory depression. Current discoveries are limited by lack of amenable animal models to screen candidate drugs. Zebrafish (Danio rerio) is an emerging animal model with high reproduction and fast development, which shares remarkable similarity in their physiology and genome to mammals. However, it is unknown whether zebrafish possesses similar opioid system, respiratory and analgesic responses to opioids than mammals. In freely-behaving larval zebrafish, fentanyl depresses the rate of respiratory mandible movements and induces analgesia, effects reversed by μ-opioid receptor antagonists. Zebrafish presents evolutionary conserved mechanisms of action of opioid drugs, also found in mammals, and constitute amenable models for phenotype-based drug discovery.
Keywords: analgesia; breathing; fentanyl; medicine; neuroscience; opioid; zebrafish.
Plain language summary
When it comes to treating severe pain, a doctor’s arsenal is somewhat limited: synthetic or natural opioids such as morphine, fentanyl or oxycodone are often one of the only options available to relieve patients. Yet these compounds can make breathing slower and shallower, quickly depriving the body of oxygen and causing death. This lethal side-effect is particularly devastating as opioids misuse has reached dangerously high levels in the United States, creating an ‘opioid epidemic’ which has claimed the lives of over 80,000 Americans in 2020. It is therefore crucial to find safer drugs that do not have this effect on breathing, but this research has been slowed down by the lack of animal models in which to study the effect of new compounds. Zebrafish are small freshwater fish that reproduce and develop fast, yet they are also remarkably genetically similar to mammals and feature a complex nervous system. However, it is not known whether the effect of opioids on zebrafish is comparable to mammals, and therefore whether these animals can be used to test new drugs for pain relief. To investigate this question, Zaig et al. exposed zebrafish larvae to fentanyl, showing that the fish then exhibited slower lower jaw movements – a sign of decreased breathing. The fish also could also tolerate a painful stimulus for longer, suggesting that this opioid does reduce pain in the animals. Together, these results point towards zebrafish and mammals sharing similar opioid responses, demonstrating that the fish could be used to test potential pain medications. The methods Zaig et al. have developed to establish these results could be harnessed to quickly assess large numbers of drug compounds, as well as decipher how pain emerges and can be stopped.
© 2021, Zaig et al.
Conflict of interest statement
SZ, Cd, GM No competing interests declared
Figures
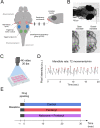
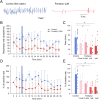



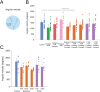




Comment in
-
Zebrafish: a new model system for the development of safe opioid therapies.Lab Anim (NY). 2021 May;50(5):123. doi: 10.1038/s41684-021-00763-6. Lab Anim (NY). 2021. PMID: 33903759 No abstract available.
Similar articles
-
ß-arrestin 2 germline knockout does not attenuate opioid respiratory depression.Elife. 2021 May 18;10:e62552. doi: 10.7554/eLife.62552. Elife. 2021. PMID: 34002697 Free PMC article.
-
Opioids depress breathing through two small brainstem sites.Elife. 2020 Feb 19;9:e52694. doi: 10.7554/eLife.52694. Elife. 2020. PMID: 32073401 Free PMC article.
-
Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone).Pain Pract. 2008 Jul-Aug;8(4):287-313. doi: 10.1111/j.1533-2500.2008.00204.x. Epub 2008 May 23. Pain Pract. 2008. PMID: 18503626
-
In vivo profiling of seven common opioids for antinociception, constipation and respiratory depression: no two opioids have the same profile.Br J Pharmacol. 2015 Jan;172(2):532-48. doi: 10.1111/bph.12696. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24641546 Free PMC article.
-
Non-analgesic effects of opioids: opioid-induced respiratory depression.Curr Pharm Des. 2012;18(37):5994-6004. doi: 10.2174/138161212803582469. Curr Pharm Des. 2012. PMID: 22747535 Review.
Cited by
-
Sleep Deficiency and Opioid Use Disorder: Trajectory, Mechanisms, and Interventions.Clin Chest Med. 2022 Jun;43(2):e1-e14. doi: 10.1016/j.ccm.2022.05.001. Clin Chest Med. 2022. PMID: 35659031 Free PMC article. Review.
-
Advances in Zebrafish as a Comprehensive Model of Mental Disorders.Depress Anxiety. 2023 Jun 20;2023:6663141. doi: 10.1155/2023/6663141. eCollection 2023. Depress Anxiety. 2023. PMID: 40224594 Free PMC article. Review.
-
Current Methods to Investigate Nociception and Pain in Zebrafish.Front Neurosci. 2021 Apr 8;15:632634. doi: 10.3389/fnins.2021.632634. eCollection 2021. Front Neurosci. 2021. PMID: 33897350 Free PMC article. Review.
-
Fingerprint Analysis and Comparison of Activity Differences of Crude Venom from Five Species of Vermivorous Cone Snail in the South China Sea.Mar Drugs. 2025 Feb 25;23(3):102. doi: 10.3390/md23030102. Mar Drugs. 2025. PMID: 40137288 Free PMC article.
-
Persistent Transcriptome Alterations in Zebrafish Embryos After Discontinued Opioid Exposure.Int J Mol Sci. 2025 May 19;26(10):4840. doi: 10.3390/ijms26104840. Int J Mol Sci. 2025. PMID: 40429979 Free PMC article.
References
-
- Avdesh A, Chen M, Martin-Iverson MT, Mondal A, Ong D, Rainey-Smith S, Taddei K, Lardelli M, Groth DM, Verdile G, Martins RN. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: an introduction. Journal of Visualized Experiments. 2012;1:e4196. doi: 10.3791/4196. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

