N-cadherin upregulation mediates adaptive radioresistance in glioblastoma
- PMID: 33720050
- PMCID: PMC7954595
- DOI: 10.1172/JCI136098
N-cadherin upregulation mediates adaptive radioresistance in glioblastoma
Abstract
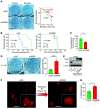
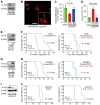
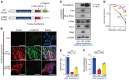
Glioblastoma (GBM) is composed of heterogeneous tumor cell populations, including those with stem cell properties, termed glioma stem cells (GSCs). GSCs are innately less radiation sensitive than the tumor bulk and are believed to drive GBM formation and recurrence after repeated irradiation. However, it is unclear how GSCs adapt to escape the toxicity of repeated irradiation used in clinical practice. To identify important mediators of adaptive radioresistance in GBM, we generated radioresistant human and mouse GSCs by exposing them to repeat cycles of irradiation. Surviving subpopulations acquired strong radioresistance in vivo, which was accompanied by a reduction in cell proliferation and an increase in cell-cell adhesion and N-cadherin expression. Increasing N-cadherin expression rendered parental GSCs radioresistant, reduced their proliferation, and increased their stemness and intercellular adhesive properties. Conversely, radioresistant GSCs lost their acquired phenotypes upon CRISPR/Cas9-mediated knockout of N-cadherin. Mechanistically, elevated N-cadherin expression resulted in the accumulation of β-catenin at the cell surface, which suppressed Wnt/β-catenin proliferative signaling, reduced neural differentiation, and protected against apoptosis through Clusterin secretion. N-cadherin upregulation was induced by radiation-induced IGF1 secretion, and the radiation resistance phenotype could be reverted with picropodophyllin, a clinically applicable blood-brain-barrier permeable IGF1 receptor inhibitor, supporting clinical translation.
Keywords: Brain cancer; Cell migration/adhesion; Oncology; Radiation therapy.
Conflict of interest statement
Figures




Similar articles
-
NONHSAT141192.2 Facilitates the Stemness and Radioresistance of Glioma Stem Cells via the Regulation of PIK3R3 and SOX2.CNS Neurosci Ther. 2025 Feb;31(2):e70279. doi: 10.1111/cns.70279. CNS Neurosci Ther. 2025. PMID: 39968701 Free PMC article.
-
Downregulated CLIP3 induces radioresistance by enhancing stemness and glycolytic flux in glioblastoma.J Exp Clin Cancer Res. 2021 Sep 6;40(1):282. doi: 10.1186/s13046-021-02077-4. J Exp Clin Cancer Res. 2021. PMID: 34488821 Free PMC article.
-
CDH5 is specifically activated in glioblastoma stemlike cells and contributes to vasculogenic mimicry induced by hypoxia.Neuro Oncol. 2013 Jul;15(7):865-79. doi: 10.1093/neuonc/not029. Epub 2013 May 3. Neuro Oncol. 2013. PMID: 23645533 Free PMC article.
-
Improving the radiosensitivity of radioresistant and hypoxic glioblastoma.Future Oncol. 2010 Oct;6(10):1591-601. doi: 10.2217/fon.10.123. Future Oncol. 2010. PMID: 21062158 Review.
-
Glioblastoma and stem cells.Neoplasma. 2008;55(5):369-74. Neoplasma. 2008. PMID: 18665745 Review.
Cited by
-
Targeting adaptive radioresistance in glioblastoma.Neuro Oncol. 2022 Jul 1;24(7):1071-1073. doi: 10.1093/neuonc/noac074. Neuro Oncol. 2022. PMID: 35323979 Free PMC article. No abstract available.
-
Altering fractionation during radiation overcomes radio-resistance in patient-derived glioblastoma cells assessed using a novel longitudinal radiation cytotoxicity assay.Radiother Oncol. 2025 Jan;202:110646. doi: 10.1016/j.radonc.2024.110646. Epub 2024 Nov 21. Radiother Oncol. 2025. PMID: 39579870
-
Retinol dehydrogenase 10 promotes epithelial-mesenchymal transition in spinal cord gliomas via PI3K/AKT pathway.Int J Immunopathol Pharmacol. 2024 Jan-Dec;38:3946320241276336. doi: 10.1177/03946320241276336. Int J Immunopathol Pharmacol. 2024. PMID: 39180753 Free PMC article.
-
Inhibitor of Wnt receptor 1 suppresses the effects of Wnt1, Wnt3a and β‑catenin on the proliferation and migration of C6 GSCs induced by low‑dose radiation.Oncol Rep. 2024 Feb;51(2):22. doi: 10.3892/or.2023.8681. Epub 2023 Dec 15. Oncol Rep. 2024. PMID: 38099414 Free PMC article.
-
The Importance of M1-and M2-Polarized Macrophages in Glioma and as Potential Treatment Targets.Brain Sci. 2023 Aug 31;13(9):1269. doi: 10.3390/brainsci13091269. Brain Sci. 2023. PMID: 37759870 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

