A Comprehensive Enhanced Recovery Pathway for Rotator Cuff Surgery Reduces Pain, Opioid Use, and Side Effects
- PMID: 33720071
- PMCID: PMC8277252
- DOI: 10.1097/CORR.0000000000001684
A Comprehensive Enhanced Recovery Pathway for Rotator Cuff Surgery Reduces Pain, Opioid Use, and Side Effects
Abstract
Background: Patients often have moderate to severe pain after rotator cuff surgery, despite receiving analgesics and nerve blocks. There are many suggested ways to improve pain after rotator cuff surgery, but the effects of adopting a pathway that includes formal patient education, a long-acting nerve block, and extensive multimodal analgesia are unclear.
Questions/purposes: (1) Does adoption of a clinical pathway incorporating patient education, a long-acting nerve block, and preemptive multimodal analgesia reduce the worst pain during the first 48 hours after surgery compared with current standard institutional practices? (2) Does adoption of the pathway reduce opioid use? (3) Does adoption of the pathway reduce side effects and improve patient-oriented outcomes?
Methods: From September 2018 to January 2020, 281 patients scheduled for arthroscopic ambulatory rotator cuff surgery were identified for this paired sequential prospective cohort study. Among patients in the control group, 177 were identified, 33% (58) were not eligible, for 11% (20) staff was not available, 56% (99) were approached, 16% (29) declined, 40% (70) enrolled, and 40% (70) were analyzed (2% [4] lost to follow-up for secondary outcomes after postoperative day 2). For patients in the pathway cohort, 104 were identified, 17% (18) were not eligible, for 11% (11) staff was not available, 72% (75) were approached, 5% (5) declined, 67% (70) enrolled, and 67% (70) were analyzed (3% [3] lost to follow-up for secondary outcomes after postoperative day 2). No patients were lost to follow-up for primary outcome; for secondary outcomes, four were lost in the control group and three in the pathway group after postoperative day 2 (p = 0.70). The initial 70 patients enrolled received routine care (control group), and in a subsequent cohort, 70 patients received care guided by a pathway (pathway group). Of the 205 eligible patients, 68% (140) were included in the analysis. This was not a study comparing two tightly defined protocols but rather a study to determine whether adoption of a pathway would alter patient outcomes. For this reason, we used a pragmatic (real-world) study design that did not specify how control patients would be treated, and it did not require that all pathway patients receive all components of the pathway. We developed the pathway in coordination with a group of surgeons and anesthesiologists who agreed to apply the pathway as much as was viewed practical for each individual patient. Patients in both groups received a brachial plexus nerve block with sedation. Major differences between the pathway and control groups were: detailed patient education regarding reasonable pain expectations with a goal of reducing opioid use (no formal educational presentation was given to the control), a long-acting nerve block using bupivacaine with dexamethasone (control patients often received shorter-acting local anesthetic without perineural dexamethasone), and preemptive multimodal analgesia including intraoperative ketamine, postoperative acetaminophen, NSAIDs, and gabapentin at bedtime, with opioids as needed (control patients received postoperative opioids but most did not get postoperative NSAIDS and no controls received gabapentin or separate prescriptions for acetaminophen). The primary outcome was the numerical rating scale (NRS) worst pain with movement 0 to 48 hours after block placement. The NRS pain score ranges from 0 (no pain) to 10 (worst pain possible). The minimum clinically important difference (MCID) [12] for NRS that was used for calculation of the study sample size was 1.3 [18], although some authors suggest 1 [13] or 2 [5] are appropriate; if we had used an MCID of 2, the sample size would have been smaller. Secondary outcomes included NRS pain scores at rest, daily opioid use (postoperative day 1, 2, 7, 14), block duration, patient-oriented pain questions (postoperative day 1, 2, 7, 14), and patient and physician adherence to pathway.
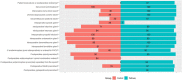
Results: On postoperative day 1, pathway patients had lower worst pain with movement (3.3 ± 3.1) compared with control patients (5.6 ± 3.0, mean difference -2.7 [95% CI -3.7 to -1.7]; p < 0.001); lower scores were also seen for pain at rest (1.9 ± 2.3 versus 4.0 ± 2.9, mean difference -2.0 [95% CI -2.8 to -1.3]; p < 0.001). Cumulative postoperative opioid use (0-48 hours) was reduced (pathway oral morphine equivalent use was 23 ± 28 mg versus 44 ± 35 mg, mean difference 21 [95% CI 10 to 32]; p < 0.01). The greatest difference in opioid use was in the first 24 hours after surgery (pathway 7 ± 12 mg versus control 21 ± 21 mg, mean difference -14 [95% CI -19 to -10]; p < 0.01). On postoperative day 1, pathway patients had less interference with staying asleep compared with control patients (0.5 ± 1.6 versus 2.6 ± 3.3, mean difference -2.2 [95% CI -3.3 to -1.1]; p < 0.001); lower scores were also seen for interference with activities (0.9 ± 2.3 versus 1.9 ± 2.9, mean difference -1.1 [95% CI -2 to -0.1]; p = 0.03). Satisfaction with pain treatment on postoperative day 1 was higher among pathway patients compared with control patients (9.2 ± 1.7 versus 8.2 ± 2.5, mean difference 1.0 [95% CI 0.3 to 1.8]; p < 0.001). On postoperative day 2, pathway patients had lower nausea scores compared with control patients (0.3 ± 1.1 versus 1 ± 2.1, mean difference -0.7 [95% CI -1.2 to -0.1]; p = 0.02); lower scores were also seen for drowsiness on postoperative day 1 (1.7 ± 2.7 versus 2.6 ± 2.6, mean difference -0.9 [95% CI - 1.7 to -0.1]; p = 0.03).
Conclusion: Adoption of the pathway was associated with improvement in the primary outcome (pain with movement) that exceeded the MCID. Patients in the pathway group had improved patient-oriented outcomes and fewer side effects. This pathway uses multiple analgesic drugs, which may pose risks to elderly patients, in particular. Therefore, in evaluating whether to use this pathway, clinicians should weigh the effect sizes against the potential risks that may emerge with large scale use, consider the difficulties involved in adapting a pathway to local practice so that pathway will persist, and recognize that this study only enrolled patients among surgeons and the anesthesiologists that advocated for the pathway; results may have been different with less enthusiastic clinicians. This pathway, based on a long-lasting nerve block, multimodal analgesia, and patient education can be considered for adoption.
Level of evidence: Level II, therapeutic study.
Trial registration: ClinicalTrials.gov NCT03717753.
Copyright © 2021 by the Association of Bone and Joint Surgeons.
Conflict of interest statement
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Figures

Comment in
-
CORR Insights®: A Comprehensive Enhanced Recovery Pathway for Rotator Cuff Surgery Reduces Pain, Opioid Use, and Side Effects.Clin Orthop Relat Res. 2021 Aug 1;479(8):1752-1753. doi: 10.1097/CORR.0000000000001746. Clin Orthop Relat Res. 2021. PMID: 33780386 Free PMC article. No abstract available.
References
-
- Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9-19. - PubMed
-
- Elkassabany NM, Wang A, Ochroch J, Mattera M, Liu J, Kuntz A. Improved quality of recovery from ambulatory shoulder surgery after implementation of a multimodal perioperative pain management protocol. Pain Med. 2019;20:1012-1019. - PubMed
-
- Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149-158. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

