Palladium-Mediated Cγ -H Functionalization of Alicyclic Amines
- PMID: 33720500
- PMCID: PMC8102420
- DOI: 10.1002/anie.202101782
Palladium-Mediated Cγ -H Functionalization of Alicyclic Amines
Abstract
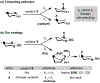
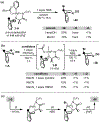
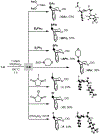
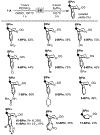
This paper describes a new method for the transannular functionalization of the γ-C-H bonds in alicyclic amines to install C(sp3 )-halogen, oxygen, nitrogen, boron, and sulfur bonds. The key challenge for this transformation is controlling the relative rate of Cγ -H versus Cα -H functionalization. We demonstrate that this selectivity can be achieved by pre-complexation of the substrate with Pd prior to the addition of oxidant. This approach enables the use of diverse oxidants that ultimately install various heteroatom functional groups at the γ-position with high site- and diastereoselectivity.
Keywords: C−H activation; alicyclic amines; palladium; relative rates.
© 2021 Wiley-VCH GmbH.
Figures
References
-
- Källström S, Leino R, Bioorg. Med. Chem. 2008, 16, 601–635. - PubMed
-
- For examples of Cα—H of alicyclic amines, see:a) Pastine SJ, Gribkov D, Sames D, J. Am. Chem. Soc. 2006, 128, 14220–14221; - PubMed
- b) Campos KR, Chem. Soc. Rev. 2007, 36, 1069–1084; - PubMed
- c) Mitchell EA, Peschiulli A, Lefevre N, Meerpoel L, Maes BUW, Chem. - Eur. J . 2012, 18, 10092–10142; - PubMed
- d) Shi L, Xia W, Chem. Soc. Rev. 2012, 41, 7687–7697; - PubMed
- e) He J, Hamann LG, Davies HML, Beckwith REJ, Nat. Commun. 2015, 6, 5943; - PubMed
- f) Spangler JE, Kobayashi Y, Verma P, Wang D-H, Yu J-Q, J. Am. Chem. Soc. 2015, 137, 11876–11879; - PMC - PubMed
- g) Chen W, Ma L, Paul A, Seidel D, Nat. Chem . 2017, 10, 165–169. - PMC - PubMed
-
- For examples of amines undergoing side reactions in presence of metal catalysts and oxidants, see:a) Murahashi S, Naota T, Yonemura K, J. Am. Chem. Soc . 1988, 110, 8256–8258;
- b) Venkataramanan NS, Kuppuraj G, Rajagopal S, Coord. Chem. Rev. 2005, 249, 1249–1268;
- c) Park J, Morimoto Y, Lee Y-M, You Y, Nam W, Fukuzumi S, Inorg. Chem. 2011, 50, 11612–11622; - PubMed
- d) Liu P, Liu Y, Wong EL-M, Xiang S, Che C-M, Chem. Sci. 2011, 2, 2187–2195;
- e) Cai XW, Sha M, Guo CP, Pan RM, Asian J Chem . 2012, 24, 3781–3784;
- f) Genovino J, Lütz S, Sames D, Touré BB, J. Am. Chem. Soc . 2013, 135, 12346–12352; - PubMed
- g) Ling Z, Yun L, Liu L, Fu X, Chem. Commun . 2013, 49, 4214–4216; - PubMed
- h) Malik HA, Taylor BLH, Kerrigan JR, Grob JE, Houk KN, Du Bois J, Hamann LG, Patterson AW, Chem. Sci . 2014, 5, 2352–2361; - PMC - PubMed
- i) Kim S, Ginsbach JW, Lee JY, Peterson RL, Liu JJ, Siegler MA, Sarjeant AA, Solomon EI, Karlin KD, J. Am. Chem. Soc. 2015, 137, 2867–2874. - PMC - PubMed
-
- Wayner DDM, Clark KB, Rauk A, Yu D, Armstrong DA, J. Am. Chem. Soc . 1997, 119, 8925–8932.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources