Linear-nonlinear cascades capture synaptic dynamics
- PMID: 33720935
- PMCID: PMC7993773
- DOI: 10.1371/journal.pcbi.1008013
Linear-nonlinear cascades capture synaptic dynamics
Abstract
Short-term synaptic dynamics differ markedly across connections and strongly regulate how action potentials communicate information. To model the range of synaptic dynamics observed in experiments, we have developed a flexible mathematical framework based on a linear-nonlinear operation. This model can capture various experimentally observed features of synaptic dynamics and different types of heteroskedasticity. Despite its conceptual simplicity, we show that it is more adaptable than previous models. Combined with a standard maximum likelihood approach, synaptic dynamics can be accurately and efficiently characterized using naturalistic stimulation patterns. These results make explicit that synaptic processing bears algorithmic similarities with information processing in convolutional neural networks.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





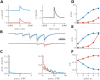
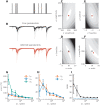
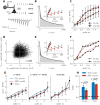

References
-
- Feng T. Studies on the neuromuscular junction. XXVI. The changes of the end-plate potential during and after prolonged stimulation. Chinese Journal of Physiology. 1941;16:341–372.
-
- Eccles JC, Katz B, Kuffler SW. Nature of the “endplate potential” in curarized muscle. Journal of Neurophysiology. 1941;4(5):362–387. 10.1152/jn.1941.4.5.362 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

