Ischemic stroke in PAR1 KO mice: Decreased brain plasmin and thrombin activity along with decreased infarct volume
- PMID: 33720950
- PMCID: PMC7959388
- DOI: 10.1371/journal.pone.0248431
Ischemic stroke in PAR1 KO mice: Decreased brain plasmin and thrombin activity along with decreased infarct volume
Abstract
Background: Ischemic stroke is a common and debilitating disease with limited treatment options. Protease activated receptor 1 (PAR1) is a fundamental cell signaling mediator in the central nervous system (CNS). It can be activated by many proteases including thrombin and plasmin, with various down-stream effects, following brain ischemia.
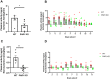
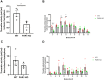
Methods: A permanent middle cerebral artery occlusion (PMCAo) model was used in PAR1 KO and WT C57BL/6J male mice. Mice were evaluated for neurological deficits (neurological severity score, NSS), infarct volume (Tetrazolium Chloride, TTC), and for plasmin and thrombin activity in brain slices.
Results: Significantly low levels of plasmin and thrombin activities were found in PAR1 KO compared to WT (1.6±0.4 vs. 3.2±0.6 ng/μl, p<0.05 and 17.2±1.0 vs. 21.2±1.0 mu/ml, p<0.01, respectively) along with a decreased infarct volume (178.9±14.3, 134.4±13.3 mm3, p<0.05).
Conclusions: PAR1 KO mice have smaller infarcts, with lower thrombin and plasmin activity levels. These findings may suggest that modulation of PAR1 is a potential target for future pharmacological treatment of ischemic stroke.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet. 2014;384: 1929–1935. 10.1016/S0140-6736(14)60584-5 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

