Innate immune signatures to a partially-efficacious HIV vaccine predict correlates of HIV-1 infection risk
- PMID: 33720973
- PMCID: PMC7959397
- DOI: 10.1371/journal.ppat.1009363
Innate immune signatures to a partially-efficacious HIV vaccine predict correlates of HIV-1 infection risk
Abstract
The pox-protein regimen tested in the RV144 trial is the only vaccine strategy demonstrated to prevent HIV-1 infection. Subsequent analyses identified antibody and cellular immune responses as correlates of risk (CoRs) for HIV infection. Early predictors of these CoRs could provide insight into vaccine-induced protection and guide efforts to enhance vaccine efficacy. Using specimens from a phase 1b trial of the RV144 regimen in HIV-1-uninfected South Africans (HVTN 097), we profiled innate responses to the first ALVAC-HIV immunization. PBMC transcriptional responses peaked 1 day post-vaccination. Type I and II interferon signaling pathways were activated, as were innate pathways critical for adaptive immune priming. We then identified two innate immune transcriptional signatures strongly associated with adaptive immune CoR after completion of the 4-dose regimen. Day 1 signatures were positively associated with antibody-dependent cellular cytotoxicity and phagocytosis activity at Month 6.5. Conversely, a signature present on Days 3 and 7 was inversely associated with Env-specific CD4+ T cell responses at Months 6.5 and 12; rapid resolution of this signature was associated with higher Env-specific CD4+ T-cell responses. These are the first-reported early immune biomarkers of vaccine-induced responses associated with HIV-1 acquisition risk in humans and suggest hypotheses to improve HIV-1 vaccine regimens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

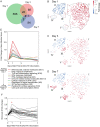
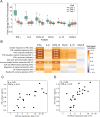

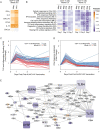
Comment in
-
Rapid transient and longer-lasting innate cytokine changes associated with adaptive immunity after repeated SARS-CoV-2 BNT162b2 mRNA vaccinations.Front Immunol. 2023 Nov 27;14:1292568. doi: 10.3389/fimmu.2023.1292568. eCollection 2023. Front Immunol. 2023. PMID: 38090597 Free PMC article.
References
-
- UNAIDS. Global HIV & AIDS statistics—2019 fact sheet. https://www.unaids.org/en/resources/fact-sheet.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

