Application of xCELLigence real-time cell analysis to the microplate assay for pertussis toxin induced clustering in CHO cells
- PMID: 33720984
- PMCID: PMC7959359
- DOI: 10.1371/journal.pone.0248491
Application of xCELLigence real-time cell analysis to the microplate assay for pertussis toxin induced clustering in CHO cells
Abstract
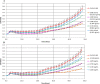
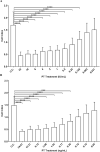
The microplate assay with Chinese Hamster Ovary (CHO) cells is currently used as a safety test to monitor the residual pertussis toxin (PT) amount in acellular pertussis antigens prior to vaccine formulation. The assay is based on the findings that the exposure of CHO cells to PT results in a concentration-dependent clustering response which can be used to estimate the amount of PT in a sample preparation. A major challenge with the current CHO cell assay methodology is that scoring of PT-induced clustering is dependent on subjective operator visual assessment using light microscopy. In this work, we have explored the feasibility of replacing the microscopy readout for the CHO cell assay with the xCELLigence Real-Time Cell Analysis system (ACEA BioSciences, a part of Agilent). The xCELLigence equipment is designed to monitor cell adhesion and growth. The electrical impedance generated from cell attachment and proliferation is quantified via gold electrodes at the bottom of the cell culture plate wells, which is then translated into a unitless readout called cell index. Results showed significant decrease in the cell index readouts of CHO cells exposed to PT compared to the cell index of unexposed CHO cells. Similar endpoint concentrations were obtained when the PT reference standard was titrated with either xCELLigence or microscopy. Testing genetically detoxified pertussis samples unspiked or spiked with PT further supported the sensitivity and reproducibility of the xCELLigence assay in comparison with the conventional microscopy assay. In conclusion, the xCELLigence RTCA system offers an alternative automated and higher throughput method for evaluating PT-induced clustering in CHO cells.
Conflict of interest statement
All authors were employees of, and may hold shares or stocks in, the commercial company Sanofi Pasteur at the time of this work. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- Dorji D, Mooi F, Yantorno O, Deora R, Graham RM, Mukkur TK. Bordetella Pertussis virulence factors in the continuing evolution of whooping cough vaccines for improved performance. Med Microbiol Immunol. 2018;207(1):3–26. - PubMed
-
- Corbel MJ, Xing DK. Toxicity and potency evaluation of pertussis vaccines. Expert Rev Vaccines. 2004;3(1):89–101. - PubMed
-
- Hsia JA, Tsai SC, Adamik R, Yost DA, Hewlett EL, Moss J. Amino acid-specific ADP-ribosylation. Sensitivity to hydroxylamine of [cysteine(ADP-ribose)]protein and [arginine(ADP-ribose)]protein linkages. J Biol Chem. 1985;260(30):16187–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

