Aerobic glycolysis supports hepatitis B virus protein synthesis through interaction between viral surface antigen and pyruvate kinase isoform M2
- PMID: 33720996
- PMCID: PMC8009439
- DOI: 10.1371/journal.ppat.1008866
Aerobic glycolysis supports hepatitis B virus protein synthesis through interaction between viral surface antigen and pyruvate kinase isoform M2
Abstract
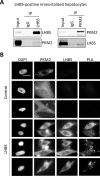
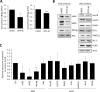
As an intracellular pathogen, the reproduction of the hepatitis B virus (HBV) depends on the occupancy of host metabolism machinery. Here we test a hypothesis if HBV may govern intracellular biosynthesis to achieve a productive reproduction. To test this hypothesis, we set up an affinity purification screen for host factors that interact with large viral surface antigens (LHBS). This identified pyruvate kinase isoform M2 (PKM2), a key regulator of glucose metabolism, as a binding partner of viral surface antigens. We showed that the expression of viral LHBS affected oligomerization of PKM2 in hepatocytes, thereby increasing glucose consumption and lactate production, a phenomenon known as aerobic glycolysis. Reduction of PKM2 activity was also validated in several different models, including HBV-infected HepG2-NTCP-C4 cells, adenovirus mediated HBV gene transduction and transfection with a plasmid containing complete HBV genome on HuH-7 cells. We found the recovery of PKM2 activity in hepatocytes by chemical activators, TEPP-46 or DASA-58, reduced expressions of viral surface and core antigens. In addition, reduction of glycolysis by culturing in low-glucose condition or treatment with 2-deoxyglucose also decreased expressions of viral surface antigen, without affecting general host proteins. Finally, TEPP-46 largely suppressed proliferation of LHBS-positive cells on 3-dimensional agarose plates, but showed no effect on the traditional 2-dimensional cell culture. Taken together, these results indicate that HBV-induced metabolic switch may support its own translation in hepatocytes. In addition, aerobic glycolysis is likely essential for LHBS-mediated oncogenesis. Accordingly, restriction of glucose metabolism may be considered as a novel strategy to restrain viral protein synthesis and subsequent oncogenesis during chronic HBV infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

