Comprehensive total evidence phylogeny of chinchillids (Rodentia, Caviomorpha): Cheek teeth anatomy and evolution
- PMID: 33721329
- PMCID: PMC8273581
- DOI: 10.1111/joa.13430
Comprehensive total evidence phylogeny of chinchillids (Rodentia, Caviomorpha): Cheek teeth anatomy and evolution
Abstract
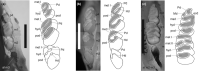
Rodents are the most diverse order of extant mammals, and caviomorph rodents, or New World hystricognaths, have a remarkable morphological disparity and a long fossil record that begins in the Eocene. Chinchilloidea is a poorly understood clade within Caviomorpha, from an evolutionary and phylogenetic perspective. It includes the extant families Chinchillidae and Dinomyidae, the extinct Neoepiblemidae and Cephalomyidae, and several extinct chinchilloids without a clear phylogenetic position, like Eoincamys, Borikenomys, Chambiramys, Ucayalimys, Incamys, Saremmys, Garridomys and Scotamys. The family Chinchillidae includes the extant Chinchilla and Lagidium, grouped in Chinchillinae, and the only living Lagostominae, Lagostomus maximus. Among extinct chinchillids, Eoviscaccia (early Oligocene-early Miocene of Argentina, Bolivia and Chile), Prolagostomus (early-middle Miocene of Argentina, Bolivia and Chile) and Pliolagostomus (early-middle Miocene of Argentina) are the only genera originally described as members of the family. Based on the study of specimens with unworn or little-worn cheek teeth, belonging to extinct and extant taxa, we propose homologies of the cheek teeth structures and perform a combined molecular and morphological phylogenetic analysis including extinct and extant taxa of all families of Chinchilloidea and all genera of Chinchillidae. Our phylogenetic analysis recovered three major lineages in the evolutionary history of Chinchilloidea. The first major lineage is composed of the extant taxa Chinchilla, Lagidium and Lagostomus, and the extinct genera Eoviscaccia, Prolagostomus, Pliolagostomus, Garridomys, Incamys, Loncolicu and Saremmys. Cephalomyid (Banderomys, Cephalomys, Litodontomys, Soriamys) and neoepiblemid (Neoepiblema, Perimys, Phoberomys, Scotamys) genera are part of the second major lineage, while dinomyids such as Dinomys, Drytomomys, Scleromys, 'Scleromys' and Tetrastylus constitute the third major lineage within Chinchilloidea. The phylogenetic position of some taxa previously considered as incertae sedis chinchilloids or without a clear suprageneric group (i.e. Incamys, Saremmys, Garridomys and Loncolicu) show that they belong to pan-Chinchillidae and conform the stem Chinchillidae along with Eoviscaccia. The euhypsodont crown Chinchillidae includes the living subfamilies Chinchillinae and Lagostominae. Dinomyidae and Eoincamys pascuali are recovered as the sisters of a major clade composed by 'Cephalomyidae'+Neopiblemidae and pan-Chinchillidae, and Chambiramys sylvaticus occupies a basal position to the same clade. Four major radiation events are identified in the evolutionary history of Chinchilloidea. The analysis of new morphological characters linked with molecular evidence as well as the addition of taxa of uncertain or unstable phylogenetic position or not considered in previous studies allowed us resolve part of the relationships within Chinchilloidea, particularly that of Chinchillidae, supporting preceding morphological hypotheses.
Keywords: Cenozoic; Chinchilloidea; South America; dental homologies; ontogeny.
© 2021 Anatomical Society.
Figures







References
-
- Álvarez, A. , Arévalo, R.L.M. & Verzi, D.H. (2017) Diversification patterns and size evolution in caviomorph rodents. Biological Journal of the Linnean Society, 121, 907–922.
-
- Ameghino, F. (1887) Enumeración sistemática de las especies de mamíferos fósiles coleccionados por Carlos Ameghino en los terrenos eocenos de Patagonia austral y depositados en el Museo de La Plata. Boletín del Museo de La Plata, 1, 1–26.
-
- Arnal, M. , Kramarz, A.G. , Vucetich, M.G. , Frailey, C.D. & Campbell, K.E. Jr (2020) New Palaeogene caviomorphs (Rodentia, Hystricognathi) from Santa Rosa, Peru: systematics, biochronology, biogeography and early evolutionary trends. Papers in Palaeontology, 6, 193–216.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

