Endothelial deletion of PKCδ prevents VEGF inhibition and restores blood flow reperfusion in diabetic ischemic limb
- PMID: 33722087
- PMCID: PMC8481738
- DOI: 10.1177/1479164121999033
Endothelial deletion of PKCδ prevents VEGF inhibition and restores blood flow reperfusion in diabetic ischemic limb
Abstract
Aims: Peripheral artery disease is a complication of diabetes leading to critical hindlimb ischemia. Diabetes-induced inhibition of VEGF actions is associated with the activation of protein kinase Cδ (PKCδ). We aim to specifically investigate the role of PKCδ in endothelial cell (EC) function and VEGF signaling.
Methods: Nondiabetic and diabetic mice, with (ec-Prkcd-/-) or without (ec-Prkcdf/f) endothelial deletion of PKCδ, underwent femoral artery ligation. Blood flow reperfusion was assessed up to 4 weeks post-surgery. Capillary density, EC apoptosis and VEGF signaling were evaluated in the ischemic muscle. Src homology region 2 domain-containing phosphatase-1 (SHP-1) phosphatase activity was assessed in vitro using primary ECs.
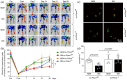
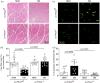
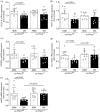
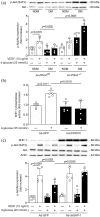
Results: Ischemic muscle of diabetic ec-Prkcdf/f mice exhibited reduced blood flow reperfusion and capillary density while apoptosis increased as compared to nondiabetic ec-Prkcdf/f mice. In contrast, blood flow reperfusion and capillary density were significantly improved in diabetic ec-Prkcd-/- mice. VEGF signaling pathway was restored in diabetic ec-Prkcd-/- mice. The deletion of PKCδ in ECs prevented diabetes-induced VEGF unresponsiveness through a reduction of SHP-1 phosphatase activity.
Conclusions: Our data provide new highlights in mechanisms by which PKCδ activation in EC contributed to poor collateral vessel formation, thus, offering novel therapeutic targets to improve angiogenesis in the diabetic limb.
Keywords: Diabetes; PKCδ; SH2 domain-containing phosphatase 1; peripheral arterial disease; vascular endothelial growth factor.
Conflict of interest statement
Figures

References
-
- American Diabetes Association. Peripheral arterial disease in people with diabetes. Clin Diabetes 2004; 22(4): 181–189.
-
- Carmeliet P.Angiogenesis in health and disease. Nat Med 2003; 9(6): 8. - PubMed
-
- Lamalice L, Le Boeuf F, Huot J.Endothelial Cell Migration During Angiogenesis. Circ Res 2007; 100(6): 782–794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

