The global burden of yellow fever
- PMID: 33722340
- PMCID: PMC7963473
- DOI: 10.7554/eLife.64670
The global burden of yellow fever
Abstract
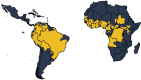
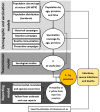
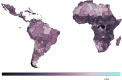
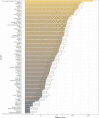
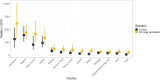
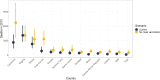
Yellow fever (YF) is a viral, vector-borne, haemorrhagic fever endemic in tropical regions of Africa and South America. The vaccine for YF is considered safe and effective, but intervention strategies need to be optimised; one of the tools for this is mathematical modelling. We refine and expand an existing modelling framework for Africa to account for transmission in South America. We fit to YF occurrence and serology data. We then estimate the subnational forces of infection for the entire endemic region. Finally, using demographic and vaccination data, we examine the impact of vaccination activities. We estimate that there were 109,000 (95% credible interval [CrI] [67,000-173,000]) severe infections and 51,000 (95% CrI [31,000-82,000]) deaths due to YF in Africa and South America in 2018. We find that mass vaccination activities in Africa reduced deaths by 47% (95% CrI [10%-77%]). This methodology allows us to evaluate the effectiveness of vaccination and illustrates the need for continued vigilance and surveillance of YF.
Keywords: epidemiology; global health; mathematical modelling; vaccine impact; vector-borne; virus; yellow fever.
© 2021, Gaythorpe et al.
Conflict of interest statement
KG, AH, KJ, DG, LC, TG, NF No competing interests declared
Figures

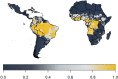
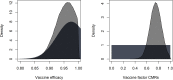
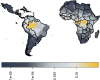
References
-
- Brady OJ, Golding N, Pigott DM, Kraemer MU, Messina JP, Reiner RC, Scott TW, Smith DL, Gething PW, Hay SI. Global temperature constraints on aedes aegypti and ae. albopictus persistence and competence for dengue virus transmission. Parasites & Vectors. 2014;7:338. doi: 10.1186/1756-3305-7-338. - DOI - PMC - PubMed
-
- Chepkorir E, Tchouassi DP, Konongoi SL, Lutomiah J, Tigoi C, Irura Z, Eyase F, Venter M, Sang R. Serological evidence of Flavivirus circulation in human populations in northern Kenya: an assessment of disease risk 2016-2017. Virology Journal. 2019;16:65. doi: 10.1186/s12985-019-1176-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

