TAK-101 Nanoparticles Induce Gluten-Specific Tolerance in Celiac Disease: A Randomized, Double-Blind, Placebo-Controlled Study
- PMID: 33722583
- PMCID: PMC9053078
- DOI: 10.1053/j.gastro.2021.03.014
TAK-101 Nanoparticles Induce Gluten-Specific Tolerance in Celiac Disease: A Randomized, Double-Blind, Placebo-Controlled Study
Abstract
Background & aims: In celiac disease (CeD), gluten induces immune activation, leading to enteropathy. TAK-101, gluten protein (gliadin) encapsulated in negatively charged poly(dl-lactide-co-glycolic acid) nanoparticles, is designed to induce gluten-specific tolerance.
Methods: TAK-101 was evaluated in phase 1 dose escalation safety and phase 2a double-blind, randomized, placebo-controlled studies. Primary endpoints included pharmacokinetics, safety, and tolerability of TAK-101 (phase 1) and change from baseline in circulating gliadin-specific interferon-γ-producing cells at day 6 of gluten challenge, in patients with CeD (phase 2a). Secondary endpoints in the phase 2a study included changes from baseline in enteropathy (villus height to crypt depth ratio [Vh:Cd]), and frequency of intestinal intraepithelial lymphocytes and peripheral gut-homing T cells.
Results: In phase 2a, 33 randomized patients completed the 14-day gluten challenge. TAK-101 induced an 88% reduction in change from baseline in interferon-γ spot-forming units vs placebo (2.01 vs 17.58, P = .006). Vh:Cd deteriorated in the placebo group (-0.63, P = .002), but not in the TAK-101 group (-0.18, P = .110), although the intergroup change from baseline was not significant (P = .08). Intraepithelial lymphocyte numbers remained equal. TAK-101 reduced changes in circulating α4β7+CD4+ (0.26 vs 1.05, P = .032), αEβ7+CD8+ (0.69 vs 3.64, P = .003), and γδ (0.15 vs 1.59, P = .010) effector memory T cells. TAK-101 (up to 8 mg/kg) induced no clinically meaningful changes in vital signs or routine clinical laboratory evaluations. No serious adverse events occurred.
Conclusions: TAK-101 was well tolerated and prevented gluten-induced immune activation in CeD. The findings from the present clinical trial suggest that antigen-specific tolerance was induced and represent a novel approach translatable to other immune-mediated diseases. ClinicalTrials.gov identifiers: NCT03486990 and NCT03738475.
Keywords: Antigen-specific Immune Tolerance; Gliadin.
Copyright © 2021 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures





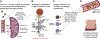
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

