MRTFA: A critical protein in normal and malignant hematopoiesis and beyond
- PMID: 33722605
- PMCID: PMC8079280
- DOI: 10.1016/j.jbc.2021.100543
MRTFA: A critical protein in normal and malignant hematopoiesis and beyond
Abstract
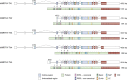
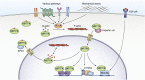
Myocardin-related transcription factor A (MRTFA) is a coactivator of serum response factor, a transcription factor that participates in several critical cellular functions including cell growth and apoptosis. MRTFA couples transcriptional regulation to actin cytoskeleton dynamics, and the transcriptional targets of the MRTFA-serum response factor complex include genes encoding cytoskeletal proteins as well as immediate early genes. Previous work has shown that MRTFA promotes the differentiation of many cell types, including various types of muscle cells and hematopoietic cells, and MRTFA's interactions with other protein partners broaden its cellular roles. However, despite being first identified as part of the recurrent t(1;22) chromosomal translocation in acute megakaryoblastic leukemia, the mechanisms by which MRTFA functions in malignant hematopoiesis have yet to be defined. In this review, we provide an in-depth examination of the structure, regulation, and known functions of MRTFA with a focus on hematopoiesis. We conclude by identifying areas of study that merit further investigation.
Keywords: G-actin; Ras homolog gene family member A; cell differentiation; cytoskeleton; hematopoiesis; leukemia; myocardin; platelet; serum response factor.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


Similar articles
-
Protein Kinase N Promotes Stress-Induced Cardiac Dysfunction Through Phosphorylation of Myocardin-Related Transcription Factor A and Disruption of Its Interaction With Actin.Circulation. 2019 Nov 19;140(21):1737-1752. doi: 10.1161/CIRCULATIONAHA.119.041019. Epub 2019 Sep 30. Circulation. 2019. PMID: 31564129
-
WAVE2 Is a Vital Regulator in Myogenic Differentiation of Progenitor Cells through the Mechanosensitive MRTFA-SRF Axis.Cells. 2023 Dec 20;13(1):9. doi: 10.3390/cells13010009. Cells. 2023. PMID: 38201213 Free PMC article.
-
MRTFA augments megakaryocyte maturation by enhancing the SRF regulatory axis.Blood Adv. 2018 Oct 23;2(20):2691-2703. doi: 10.1182/bloodadvances.2018019448. Blood Adv. 2018. PMID: 30337297 Free PMC article.
-
Myocardin/MKL family of SRF coactivators: key regulators of immediate early and muscle specific gene expression.J Cell Biochem. 2004 Sep 1;93(1):74-82. doi: 10.1002/jcb.20199. J Cell Biochem. 2004. PMID: 15352164 Review.
-
Serum response factor-cofactor interactions and their implications in disease.FEBS J. 2021 May;288(10):3120-3134. doi: 10.1111/febs.15544. Epub 2020 Sep 12. FEBS J. 2021. PMID: 32885587 Free PMC article. Review.
Cited by
-
An injury-responsive Rac-to-Rho GTPase switch drives activation of muscle stem cells through rapid cytoskeletal remodeling.Cell Stem Cell. 2022 Jun 2;29(6):933-947.e6. doi: 10.1016/j.stem.2022.04.016. Epub 2022 May 20. Cell Stem Cell. 2022. PMID: 35597234 Free PMC article.
-
Advances in molecular characterization of pediatric acute megakaryoblastic leukemia not associated with Down syndrome; impact on therapy development.Front Cell Dev Biol. 2023 Jun 1;11:1170622. doi: 10.3389/fcell.2023.1170622. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37325571 Free PMC article. Review.
-
MKL/SRF and Bcl6 mutual transcriptional repression safeguards the fate and positioning of neocortical progenitor cells mediated by RhoA.Sci Adv. 2023 Nov 17;9(46):eadd0676. doi: 10.1126/sciadv.add0676. Epub 2023 Nov 15. Sci Adv. 2023. PMID: 37967194 Free PMC article.
-
Relieving DYRK1A repression of MKL1 confers an adult-like phenotype to human infantile megakaryocytes.J Clin Invest. 2022 Oct 3;132(19):e154839. doi: 10.1172/JCI154839. J Clin Invest. 2022. PMID: 35925681 Free PMC article.
-
MRTF-A gain-of-function in mice impairs homeostatic renewal of the intestinal epithelium.Cell Death Dis. 2023 Sep 28;14(9):639. doi: 10.1038/s41419-023-06158-4. Cell Death Dis. 2023. PMID: 37770456 Free PMC article.
References
-
- Ma Z., Morris S.W., Valentine V., Li M., Herbrick J.A., Cui X., Bouman D., Li Y., Mehta P.K., Nizetic D., Kaneko Y., Chan G.C., Chan L.C., Squire J., Scherer S.W. Fusion of two novel genes, RBM15 and MKL1, in the t(1;22)(p13;q13) of acute megakaryoblastic leukemia. Nat. Genet. 2001;28:220–221. - PubMed
-
- Mercher T., Coniat M.B., Monni R., Mauchauffe M., Nguyen Khac F., Gressin L., Mugneret F., Leblanc T., Dastugue N., Berger R., Bernard O.A. Involvement of a human gene related to the Drosophila spen gene in the recurrent t(1;22) translocation of acute megakaryocytic leukemia. Proc. Natl. Acad. Sci. U. S. A. 2001;98:5776–5779. - PMC - PubMed
-
- Du K.L., Chen M., Li J., Lepore J.J., Mericko P., Parmacek M.S. Megakaryoblastic leukemia factor-1 transduces cytoskeletal signals and induces smooth muscle cell differentiation from undifferentiated embryonic stem cells. J. Biol. Chem. 2004;279:17578–17586. - PubMed
-
- Gilles L., Bluteau D., Boukour S., Chang Y., Zhang Y., Robert T., Dessen P., Debili N., Bernard O.A., Vainchenker W., Raslova H. MAL/SRF complex is involved in platelet formation and megakaryocyte migration by regulating MYL9 (MLC2) and MMP9. Blood. 2009;114:4221–4232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

