Resveratrol-mediated attenuation of superantigen-driven acute respiratory distress syndrome is mediated by microbiota in the lungs and gut
- PMID: 33722710
- PMCID: PMC10116750
- DOI: 10.1016/j.phrs.2021.105548
Resveratrol-mediated attenuation of superantigen-driven acute respiratory distress syndrome is mediated by microbiota in the lungs and gut
Abstract
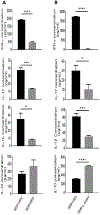
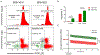
Acute Respiratory Distress Syndrome (ARDS) is triggered by a variety of agents, including Staphylococcal Enterotoxin B (SEB). Interestingly, a significant proportion of patients with COVID-19, also develop ARDS. In the absence of effective treatments, ARDS results in almost 40% mortality. Previous studies from our laboratory demonstrated that resveratrol (RES), a stilbenoid, with potent anti-inflammatory properties can attenuate SEB-induced ARDS. In the current study, we investigated the role of RES-induced alterations in the gut and lung microbiota in the regulation of ARDS. Our studies revealed that SEB administration induced inflammatory cytokines, ARDS, and 100% mortality in C3H/HeJ mice. Additionally, SEB caused a significant increase in pathogenic Proteobacteria phylum and Propionibacterium acnes species in the lungs. In contrast, RES treatment attenuated SEB-mediated ARDS and mortality in mice, and significantly increased probiotic Actinobacteria phylum, Tenericutes phylum, and Lactobacillus reuteri species in both the colon and lungs. Colonic Microbiota Transplantation (CMT) from SEB-injected mice that were treated with RES as well as the transfer of L. reuteri into recipient mice inhibited the production of SEB-mediated induction of pro-inflammatory cytokines such as IFN-γ and IL-17 but increased that of anti-inflammatory IL-10. Additionally, such CMT and L. reuteri recipient mice exposed to SEB, showed a decrease in lung-infiltrating mononuclear cells, cytotoxic CD8+ T cells, NKT cells, Th1 cells, and Th17 cells, but an increase in the population of regulatory T cells (Tregs) and Th3 cells, and increase in the survival of mice from SEB-mediated ARDS. Together, the current study demonstrates that ARDS induced by SEB triggers dysbiosis in the lungs and gut and that attenuation of ARDS by RES may be mediated, at least in part, by alterations in microbiota in the lungs and the gut, especially through the induction of beneficial bacteria such as L. reuteri.
Keywords: ARDS; Cytokine storm; DiOC(6)(3) (3,3′-Dihexyloxacarbocyanine iodide, PubChem CID: 9894321); Microbiota; Resveratrol; SEB; Superantigen; butyric acid (PubChem CID: 264); carboxymethylcellulose (PubCID: 24748); interleukin 1beta (PubChem CID: 159483); iso butyric acid (PubChem CID: 6590) and acetic acid (PubChem CID: 176); lipopolysaccharide (LPS, PubChem CID: 451715); metronidazole (Bioxtra, PubChem CID: 24896667); propionic acid (PubChem CID: 1032); resveratrol (PubChem CID: 445154).
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest in this study.
Figures



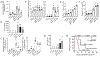
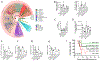
Similar articles
-
Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome through Modulation of Microbiome in the Gut-Lung Axis.Cells. 2021 Nov 25;10(12):3305. doi: 10.3390/cells10123305. Cells. 2021. PMID: 34943813 Free PMC article.
-
Protective effects of Δ9 -tetrahydrocannabinol against enterotoxin-induced acute respiratory distress syndrome are mediated by modulation of microbiota.Br J Pharmacol. 2020 Nov;177(22):5078-5095. doi: 10.1111/bph.15226. Epub 2020 Oct 8. Br J Pharmacol. 2020. PMID: 32754917 Free PMC article.
-
Resveratrol protects mice against SEB-induced acute lung injury and mortality by miR-193a modulation that targets TGF-β signalling.J Cell Mol Med. 2018 May;22(5):2644-2655. doi: 10.1111/jcmm.13542. Epub 2018 Mar 7. J Cell Mol Med. 2018. PMID: 29512867 Free PMC article.
-
Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms.Pharmacol Res. 2021 Jan;163:105224. doi: 10.1016/j.phrs.2020.105224. Epub 2020 Sep 29. Pharmacol Res. 2021. PMID: 33007416 Free PMC article. Review.
-
Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions.Pharmacol Res. 2019 Sep;147:104367. doi: 10.1016/j.phrs.2019.104367. Epub 2019 Jul 22. Pharmacol Res. 2019. PMID: 31344423 Review.
Cited by
-
Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome through Modulation of Microbiome in the Gut-Lung Axis.Cells. 2021 Nov 25;10(12):3305. doi: 10.3390/cells10123305. Cells. 2021. PMID: 34943813 Free PMC article.
-
Gut and Cutaneous Microbiome Featuring Abundance of Lactobacillus reuteri Protected Against Psoriasis-Like Inflammation in Mice.J Inflamm Res. 2021 Nov 24;14:6175-6190. doi: 10.2147/JIR.S337031. eCollection 2021. J Inflamm Res. 2021. PMID: 34853526 Free PMC article.
-
Gut microbiota modulates lung fibrosis severity following acute lung injury in mice.Commun Biol. 2022 Dec 22;5(1):1401. doi: 10.1038/s42003-022-04357-x. Commun Biol. 2022. PMID: 36543914 Free PMC article.
-
Apoptotic Vesicles Attenuate Acute Lung Injury via CD73-Mediated Inhibition of Platelet Activation and NETosis.Int J Nanomedicine. 2025 Jan 4;20:91-107. doi: 10.2147/IJN.S485012. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39802376 Free PMC article.
-
Targeting AhR as a Novel Therapeutic Modality against Inflammatory Diseases.Int J Mol Sci. 2021 Dec 28;23(1):288. doi: 10.3390/ijms23010288. Int J Mol Sci. 2021. PMID: 35008717 Free PMC article. Review.
References
-
- Nadeem A, Al-Harbi NO, Ahmad SF, Al-Harbi MM, Alhamed AS, Alfardan AS, Assiri MA, Ibrahim KE, Albassam H, Blockade of interleukin-2-inducible T-cell kinase signaling attenuates acute lung injury in mice through adjustment of pulmonary Th17/Treg immune responses and reduction of oxidative stress, Int. Immunopharmacol 83 (2020), 106369, 10.1016/j.intimp.2020.106369. - DOI - PubMed
-
- Rieder SA, Nagarkatti P, Nagarkatti M, CD1d-independent activation of invariant natural killer T cells by staphylococcal enterotoxin B through major histocompatibility complex class II/T cell receptor interaction results in acute lung injury, Infect. Immun 79 (8) (2011) 3141–3148, 10.1128/IAI.00177-11. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

