PPARG in osteocytes controls sclerostin expression, bone mass, marrow adiposity and mediates TZD-induced bone loss
- PMID: 33722775
- PMCID: PMC8076091
- DOI: 10.1016/j.bone.2021.115913
PPARG in osteocytes controls sclerostin expression, bone mass, marrow adiposity and mediates TZD-induced bone loss
Abstract
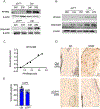
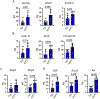
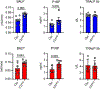
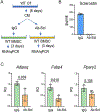
The peroxisome proliferator activated receptor gamma (PPARG) nuclear receptor regulates energy metabolism and insulin sensitivity. In this study, we present novel evidence for an essential role of PPARG in the regulation of osteocyte function, and support for the emerging concept of the conjunction between regulation of energy metabolism and bone mass. We report that PPARG is essential for sclerostin production, a recently approved target to treat osteoporosis. Our mouse model of osteocyte-specific PPARG deletion (Dmp1CrePparγflfl or γOTKO) is characterized with increased bone mass and reduced bone marrow adiposity, which is consistent with upregulation of WNT signaling and increased bone forming activity of endosteal osteoblasts. An analysis of osteocytes derived from γOTKO and control mice showed an excellent correlation between PPARG and SOST/sclerostin at the transcript and protein levels. The 8 kb sequence upstream of Sost gene transcription start site possesses multiple PPARG binding elements (PPREs) with at least two of them binding PPARG with dynamics reflecting its activation with full agonist rosiglitazone and correlating with increased levels of Sost transcript and sclerostin protein expression (Pearson's r = 0.991, p = 0.001). Older γOTKO female mice are largely protected from TZD-induced bone loss providing proof of concept that PPARG in osteocytes can be pharmacologically targeted. These findings demonstrate that transcriptional activities of PPARG are essential for sclerostin expression in osteocytes and support consideration of targeting PPARG activities with selective modulators to treat osteoporosis.
Keywords: Bone mass; Marrow adipocytes; PPARG; PPRE; SOST/sclerostin.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure
All authors state that they have no conflicts of interest.
Figures






Similar articles
-
Regulatory Effect of Osteocytes on Extramedullary and Bone Marrow Adipose Tissue Development and Function.Curr Osteoporos Rep. 2024 Jun;22(3):301-307. doi: 10.1007/s11914-024-00871-5. Epub 2024 Apr 16. Curr Osteoporos Rep. 2024. PMID: 38625510 Free PMC article. Review.
-
PPARG in osteocytes controls cell bioenergetics and systemic energy metabolism independently of sclerostin levels in circulation.Mol Metab. 2024 Oct;88:102000. doi: 10.1016/j.molmet.2024.102000. Epub 2024 Jul 27. Mol Metab. 2024. PMID: 39074536 Free PMC article.
-
PPARG in osteocytes controls cell bioenergetics and systemic energy metabolism independently of sclerostin levels in circulation.bioRxiv [Preprint]. 2024 Apr 8:2024.04.04.588029. doi: 10.1101/2024.04.04.588029. bioRxiv. 2024. Update in: Mol Metab. 2024 Oct;88:102000. doi: 10.1016/j.molmet.2024.102000. PMID: 38645043 Free PMC article. Updated. Preprint.
-
CYR61/CCN1 Regulates Sclerostin Levels and Bone Maintenance.J Bone Miner Res. 2018 Jun;33(6):1076-1089. doi: 10.1002/jbmr.3394. Epub 2018 Mar 5. J Bone Miner Res. 2018. PMID: 29351359 Free PMC article.
-
Sclerostin: From Molecule to Clinical Biomarker.Int J Mol Sci. 2022 Apr 26;23(9):4751. doi: 10.3390/ijms23094751. Int J Mol Sci. 2022. PMID: 35563144 Free PMC article. Review.
Cited by
-
Network Pharmacology and Molecular Docking Elucidate the Pharmacological Mechanism of the OSTEOWONDER Capsule for Treating Osteoporosis.Front Genet. 2022 Feb 28;13:833027. doi: 10.3389/fgene.2022.833027. eCollection 2022. Front Genet. 2022. PMID: 35295951 Free PMC article.
-
The Analysis of ECE1 and PPARG Variants in the Development of Osteopenia and Osteoporosis in Postmenopausal Women.Biomedicines. 2024 Jun 27;12(7):1440. doi: 10.3390/biomedicines12071440. Biomedicines. 2024. PMID: 39062013 Free PMC article.
-
PTH receptor signalling, osteocytes and bone disease induced by diabetes mellitus.Nat Rev Endocrinol. 2024 Nov;20(11):661-672. doi: 10.1038/s41574-024-01014-7. Epub 2024 Jul 17. Nat Rev Endocrinol. 2024. PMID: 39020007 Review.
-
The osteocyte as a signaling cell.Physiol Rev. 2022 Jan 1;102(1):379-410. doi: 10.1152/physrev.00043.2020. Epub 2021 Aug 2. Physiol Rev. 2022. PMID: 34337974 Free PMC article. Review.
-
Regulatory Effect of Osteocytes on Extramedullary and Bone Marrow Adipose Tissue Development and Function.Curr Osteoporos Rep. 2024 Jun;22(3):301-307. doi: 10.1007/s11914-024-00871-5. Epub 2024 Apr 16. Curr Osteoporos Rep. 2024. PMID: 38625510 Free PMC article. Review.
References
-
- Schwartz AV, Diabetes, bone and glucose-lowering agents: clinical outcomes, Diabetologia 60(7) (2017) 1170–1179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

