Identification and Characterization of Mycobacterium smegmatis and Mycobacterium avium subsp. paratuberculosis Zinc Transporters
- PMID: 33722846
- PMCID: PMC8117522
- DOI: 10.1128/JB.00049-21
Identification and Characterization of Mycobacterium smegmatis and Mycobacterium avium subsp. paratuberculosis Zinc Transporters
Abstract
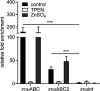
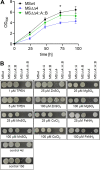
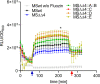
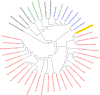
Zinc uptake in bacteria is essential to maintain cellular homeostasis and survival. ZnuABC is an important zinc importer of numerous bacterial genera, which is expressed to restore zinc homeostasis when the cytosolic concentration decreases beyond a critical threshold. Upon zinc limitation the fast-growing nonpathogenic organism Mycobacterium smegmatis (MSMEG) as well as the ruminant pathogen M. avium subsp. paratuberculosis (MAP) increases expression of genes encoding ZnuABC homologues, but also of genes encoding other transporters. This suggests an involvement of these transporters in zinc homeostasis. Here we characterized the putative zinc transporters of MSMEG (ZnuABC and ZnuABC2) and MAP (ZnuABC, MptABC, and MAP3774-76). Deletion of either ZnuABC or ZnuABC2 in MSMEG did not lead to growth defects, but to an increased expression of zinc marker genes in MSMEGΔznuABC, indicating cytosolic zinc limitation. However, chromatin immunoprecipitation proved direct binding of the global zinc regulator Zur to promoter regions of both znuABC and znuABC2. Simultaneous deletion of both transporters caused severe growth defects, which could be restored either by homologous complementation with single ZnuABC transporters or supplementation of growth media with zinc but not iron, manganese, cobalt, or magnesium. Heterologous complementation of the double mutant with MAP transporters also resulted in reconstitution of growth. Nonradioactive FluoZinTM-3AM zinc uptake assays directly revealed the competence of all transporters to import zinc. Finally, structural and phylogenetic analyses provided evidence of a novel class of ZnuABC transporters represented by the ZnuABC2 of MSMEG, which is present only in actinobacteria, mainly in the genera Nocardia, Streptomyces and fast growing Mycobacteria IMPORTANCEZinc is necessary for bacterial growth but simultaneously toxic when in excess. Hence, bacterial cells have developed systems to alter intracellular concentration. Regulation of these systems is primarily executed at transcriptional level by regulator proteins which sense femtomolar changes in the zinc level. In environmental and pathogenic mycobacteria zinc starvation induces expression of common zinc import systems such as the ZnuABC transporter, but also of other additional not yet characterized transport systems. In this study, we characterized the role of such systems in zinc transport. We showed that transport systems of both species whose transcription is induced upon zinc starvation can exchangeably restore cellular zinc homeostasis in transporter deficient mutants by transporting zinc into the cell.
Copyright © 2021 American Society for Microbiology.
Figures



References
-
- Mertens J, Smolders E. 2013. Zinc, p 465–493. In Alloway BJ (ed), Heavy metals in soils: trace metals and metalloids in soils and their bioavailability, 3rd ed, vol 22. Springer, Dordrecht, The Netherlands.
LinkOut - more resources
Full Text Sources
Other Literature Sources

