BRAF V600E/V600K Mutations versus Nonstandard Alterations: Prognostic Implications and Therapeutic Outcomes
- PMID: 33722853
- PMCID: PMC9264327
- DOI: 10.1158/1535-7163.MCT-20-0861
BRAF V600E/V600K Mutations versus Nonstandard Alterations: Prognostic Implications and Therapeutic Outcomes
Abstract
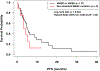
BRAF and MEK inhibitors are standard of care for BRAF V600E/K-mutated melanoma, but the benefit of BRAF and/or MEK inhibitors for nonstandard BRAF alterations for melanoma and other cancers is unclear. Patients with diverse malignancies whose cancers had undergone next-generation sequencing were screened for BRAF alterations. Demographics, treatment with BRAF and/or MEK inhibitors, clinical response, progression-free survival (PFS), and overall survival (OS) were determined from review of the electronic medical records for patients with standard BRAF V600E/K versus nonstandard BRAF alterations. A total of 213 patients with BRAF alterations (87 with nonstandard alterations) were identified; OS from diagnosis was significantly worse with nonstandard BRAF versus standard alterations, regardless of therapy [HR (95% confidence interval), 0.58 (0.38-0.88); P = 0.01]. Overall, 45 patients received BRAF/MEK-directed therapy (eight with nonstandard alterations); there were no significant differences in clinical benefit rate [stable disease ≥6 months/partial/complete response (74% vs. 63%; P = 0.39) or PFS (P = 0.24; BRAF V600E/K vs. others)]. In conclusion, patients with nonstandard versus standard BRAF alterations (BRAF V600E/K) have a worse prognosis with shorter survival from diagnosis. Even so, 63% of patients with nonstandard BRAF alterations achieved clinical benefit with BRAF/MEK inhibitors. Larger prospective studies are warranted to better understand the prognostic versus predictive implication of standard versus nonstandard BRAF alterations.
©2021 American Association for Cancer Research.
Figures
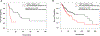

References
-
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology 2013;45:346–56. - PubMed
-
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949–54. - PubMed
-
- Turski ML, Vidwans SJ, Janku F, Garrido-Laguna I, Munoz J, Schwab R, et al. Genomically Driven Tumors and Actionability across Histologies: BRAF-Mutant Cancers as a Paradigm. Mol Cancer Ther 2016;15:533–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

