Myosin-X is dispensable for spindle morphogenesis and positioning in the mouse oocyte
- PMID: 33722900
- PMCID: PMC8077531
- DOI: 10.1242/dev.199364
Myosin-X is dispensable for spindle morphogenesis and positioning in the mouse oocyte
Abstract
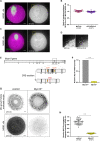
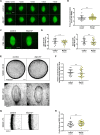
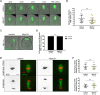
Off-center spindle positioning in mammalian oocytes enables asymmetric divisions in size, which are important for subsequent embryogenesis. The migration of the meiosis I spindle from the oocyte center to its cortex is mediated by F-actin. Specifically, an F-actin cage surrounds the microtubule spindle and applies forces to it. To better understand how F-actin transmits forces to the spindle, we studied a potential direct link between F-actin and microtubules. For this, we tested the implication of myosin-X, a known F-actin and microtubule binder involved in spindle morphogenesis and/or positioning in somatic cells, amphibian oocytes and embryos. Using a mouse strain conditionally invalidated for myosin-X in oocytes and by live-cell imaging, we show that myosin-X is not localized on the spindle, and is dispensable for spindle and F-actin assembly. It is not required for force transmission as spindle migration and chromosome alignment occur normally. More broadly, myosin-X is dispensable for oocyte developmental potential and female fertility. We therefore exclude a role for myosin-X in transmitting F-actin-mediated forces to the spindle, opening new perspectives regarding this mechanism in mouse oocytes, which differ from most mitotic cells.
Keywords: F-actin; Mouse oocyte; Myosin-X; Spindle positioning.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

Similar articles
-
Actin-based spindle positioning: new insights from female gametes.J Cell Sci. 2014 Feb 1;127(Pt 3):477-83. doi: 10.1242/jcs.142711. Epub 2014 Jan 10. J Cell Sci. 2014. PMID: 24413163 Review.
-
A new model for asymmetric spindle positioning in mouse oocytes.Curr Biol. 2008 Dec 23;18(24):1986-92. doi: 10.1016/j.cub.2008.11.022. Epub 2008 Dec 8. Curr Biol. 2008. PMID: 19062278
-
Microtubule organizing centers regulate spindle positioning in mouse oocytes.Dev Cell. 2022 Jan 24;57(2):197-211.e3. doi: 10.1016/j.devcel.2021.12.011. Epub 2022 Jan 13. Dev Cell. 2022. PMID: 35030327 Free PMC article.
-
A narrow window of cortical tension guides asymmetric spindle positioning in the mouse oocyte.Nat Commun. 2015 Jan 19;6:6027. doi: 10.1038/ncomms7027. Nat Commun. 2015. PMID: 25597399
-
Functions of actin in mouse oocytes at a glance.J Cell Sci. 2018 Nov 22;131(22):jcs218099. doi: 10.1242/jcs.218099. J Cell Sci. 2018. PMID: 30467138 Review.
Cited by
-
Antioxidant procyanidin B2 protects oocytes against cryoinjuries via mitochondria regulated cortical tension.J Anim Sci Biotechnol. 2022 Aug 16;13(1):95. doi: 10.1186/s40104-022-00742-y. J Anim Sci Biotechnol. 2022. PMID: 35971139 Free PMC article.
-
Folliculogenesis: A Cellular Crosstalk Mechanism.Curr Issues Mol Biol. 2025 Feb 10;47(2):113. doi: 10.3390/cimb47020113. Curr Issues Mol Biol. 2025. PMID: 39996834 Free PMC article. Review.
-
MYO10 promotes transzonal projection-dependent germ line-somatic contact during mammalian folliculogenesis†.Biol Reprod. 2022 Aug 9;107(2):474-487. doi: 10.1093/biolre/ioac078. Biol Reprod. 2022. PMID: 35470858 Free PMC article.
-
Filopodia-like protrusions of adjacent somatic cells shape the developmental potential of oocytes.Life Sci Alliance. 2023 Mar 21;6(6):e202301963. doi: 10.26508/lsa.202301963. Print 2023 Jun. Life Sci Alliance. 2023. PMID: 36944420 Free PMC article.
-
Follicular fluid C3a-peptide promotes oocyte maturation through F-actin aggregation.BMC Biol. 2023 Dec 8;21(1):285. doi: 10.1186/s12915-023-01760-6. BMC Biol. 2023. PMID: 38066646 Free PMC article.
References
-
- Bennabi, I., Crozet, F., Nikalayevich, E., Chaigne, A., Letort, G., Manil-Ségalen, M., Campillo, C., Cadart, C., Othmani, A., Attia, R.et al. (2020). Artificially decreasing cortical tension generates aneuploidy in mouse oocytes. Nat. Commun. 11, 1649. 10.1038/s41467-020-15470-y - DOI - PMC - PubMed
-
- Berg, J. S., Derfler, B. H., Pennisi, C. M., Corey, D. P. and Cheney, R. E. (2000). Myosin-X, a novel myosin with pleckstrin homology domains, associates with regions of dynamic actin. J. Cell Sci. 113, 3439-3451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

