Natural killer T cell immunotherapy combined with oncolytic vesicular stomatitis virus or reovirus treatments differentially increases survival in mouse models of ovarian and breast cancer metastasis
- PMID: 33722907
- PMCID: PMC7970295
- DOI: 10.1136/jitc-2020-002096
Natural killer T cell immunotherapy combined with oncolytic vesicular stomatitis virus or reovirus treatments differentially increases survival in mouse models of ovarian and breast cancer metastasis
Abstract
Background: Oncolytic viruses reduce tumor burden in animal models and have generated promising results in clinical trials. However, it is likely that oncolytic viruses will be more effective when used in combination with other therapies. Current therapeutic approaches, including chemotherapeutics, come with dose-limiting toxicities. Another option is to combine oncolytic viruses with immunotherapeutic approaches.
Methods: Using experimental models of metastatic 4T1 breast cancer and ID8 ovarian peritoneal carcinomatosis, we examined natural killer T (NKT) cell-based immunotherapy in combination with recombinant oncolytic vesicular stomatitis virus (VSV) or reovirus. 4T1 mammary carcinoma cells or ID8 ovarian cancer cells were injected into syngeneic mice. Tumor-bearing mice were treated with VSV or reovirus followed by activation of NKT cells via the intravenous administration of autologous dendritic cells loaded with the glycolipid antigen α-galactosylceramide. The effects of VSV and reovirus on immunogenic cell death (ICD), cell viability and immunogenicity were tested in vitro.
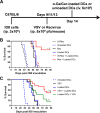
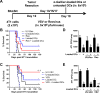
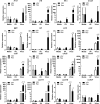
Results: VSV or reovirus treatments followed by NKT cell activation mediated greater survival in the ID8 model than individual therapies. The regimen was less effective when the treatment order was reversed, delivering virus treatments after NKT cell activation. In the 4T1 model, VSV combined with NKT cell activation increased overall survival and decreased metastatic burden better than individual treatments. In contrast, reovirus was not effective on its own or in combination with NKT cell activation. In vitro, VSV killed a panel of tumor lines better than reovirus. VSV infection also elicited greater increases in mRNA transcripts for proinflammatory cytokines, chemokines, and antigen presentation machinery compared with reovirus. Oncolytic VSV also induced the key hallmarks of ICD (calreticulin mobilization, plus release of ATP and HMGB1), while reovirus only mobilized calreticulin.
Conclusion: Taken together, these results demonstrate that oncolytic VSV and NKT cell immunotherapy can be effectively combined to decrease tumor burden in models of metastatic breast and ovarian cancers. Oncolytic VSV and reovirus induced differential responses in our models which may relate to differences in virus activity or tumor susceptibility.
Keywords: cytotoxicity; dendritic cells; immunologic; immunotherapy; natural killer T-Cells; oncolytic virotherapy.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

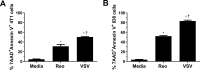

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
