ZNF91 deletion in human embryonic stem cells leads to ectopic activation of SVA retrotransposons and up-regulation of KRAB zinc finger gene clusters
- PMID: 33722937
- PMCID: PMC8015857
- DOI: 10.1101/gr.265348.120
ZNF91 deletion in human embryonic stem cells leads to ectopic activation of SVA retrotransposons and up-regulation of KRAB zinc finger gene clusters
Abstract
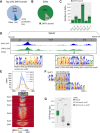
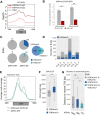
Transposable element (TE) invasions have shaped vertebrate genomes over the course of evolution. They have contributed an extra layer of species-specific gene regulation by providing novel transcription factor binding sites. In humans, SINE-VNTR-Alu (SVA) elements are one of three still active TE families; approximately 2800 SVA insertions exist in the human genome, half of which are human-specific. TEs are often silenced by KRAB zinc finger (KZNF) proteins recruiting corepressor proteins that establish a repressive chromatin state. A number of KZNFs have been reported to bind SVAs, but their individual contribution to repressing SVAs and their roles in suppressing SVA-mediated gene-regulatory effects remains elusive. We analyzed the genome-wide binding profile for ZNF91 in human cells and found that ZNF91 interacts with the VNTR region of SVAs. Through CRISPR-Cas9-mediated deletion of ZNF91 in human embryonic stem cells, we established that loss of ZNF91 results in increased transcriptional activity of SVAs. In contrast, SVA activation was not observed upon genetic deletion of the ZNF611 gene encoding another strong SVA interactor. Epigenetic profiling confirmed the loss of SVA repression in the absence of ZNF91 and revealed that mainly evolutionary young SVAs gain gene activation-associated epigenetic modifications. Genes close to activated SVAs showed a mild up-regulation, indicating SVAs adopt properties of cis-regulatory elements in the absence of repression. Notably, genome-wide derepression of SVAs elicited the communal up-regulation of KZNFs that reside in KZNF clusters. This phenomenon may provide new insights into the potential mechanisms used by the host genome to sense and counteract TE invasions.
© 2021 Haring et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
