Toxoplasma gondii association with host mitochondria requires key mitochondrial protein import machinery
- PMID: 33723040
- PMCID: PMC7999873
- DOI: 10.1073/pnas.2013336118
Toxoplasma gondii association with host mitochondria requires key mitochondrial protein import machinery
Abstract
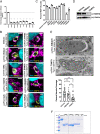
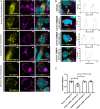
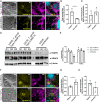
Host mitochondrial association (HMA) is a well-known phenomenon during Toxoplasma gondii infection of the host cell. The T. gondii locus mitochondrial association factor 1 (MAF1) is required for HMA and MAF1 encodes distinct paralogs of secreted dense granule effector proteins, some of which mediate the HMA phenotype (MAF1b paralogs drive HMA; MAF1a paralogs do not). To identify host proteins required for MAF1b-mediated HMA, we performed unbiased, label-free quantitative proteomics on host cells infected with type II parasites expressing MAF1b, MAF1a, and an HMA-incompetent MAF1b mutant. Across these samples, we identified ∼1,360 MAF1-interacting proteins, but only 13 that were significantly and uniquely enriched in MAF1b pull-downs. The gene products include multiple mitochondria-associated proteins, including those that traffic to the mitochondrial outer membrane. Based on follow-up endoribonuclease-prepared short interfering RNA (esiRNA) experiments targeting these candidate MAF1b-targeted host factors, we determined that the mitochondrial receptor protein TOM70 and mitochondria-specific chaperone HSPA9 were essential mediators of HMA. Additionally, the enrichment of TOM70 at the parasitophorous vacuole membrane interface suggests parasite-driven sequestration of TOM70 by the parasite. These results show that the interface between the T. gondii vacuole and the host mitochondria is characterized by interactions between a single parasite effector and multiple target host proteins, some of which are critical for the HMA phenotype itself. The elucidation of the functional members of this complex will permit us to explain the link between HMA and changes in the biology of the host cell.
Keywords: Toxoplasma gondii; mitochondria; neofunctionalization; tandem gene expansion; virulence.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Matsumoto A., Bessho H., Uehira K., Suda T., Morphological studies of the association of mitochondria with chlamydial inclusions and the fusion of chlamydial inclusions. J. Electron Microsc. (Tokyo) 40, 356–363 (1991). - PubMed
-
- Dubey J. P., Sreekumar C., Redescription of Hammondia hammondi and its differentiation from Toxoplasma gondii. Int. J. Parasitol. 33, 1437–1453 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

