Neuronal circuits overcome imbalance in excitation and inhibition by adjusting connection numbers
- PMID: 33723048
- PMCID: PMC8000583
- DOI: 10.1073/pnas.2018459118
Neuronal circuits overcome imbalance in excitation and inhibition by adjusting connection numbers
Abstract
The interplay between excitation and inhibition is crucial for neuronal circuitry in the brain. Inhibitory cell fractions in the neocortex and hippocampus are typically maintained at 15 to 30%, which is assumed to be important for stable dynamics. We have studied systematically the role of precisely controlled excitatory/inhibitory (E/I) cellular ratios on network activity using mice hippocampal cultures. Surprisingly, networks with varying E/I ratios maintain stable bursting dynamics. Interburst intervals remain constant for most ratios, except in the extremes of 0 to 10% and 90 to 100% inhibitory cells. Single-cell recordings and modeling suggest that networks adapt to chronic alterations of E/I compositions by balancing E/I connectivity. Gradual blockade of inhibition substantiates the agreement between the model and experiment and defines its limits. Combining measurements of population and single-cell activity with theoretical modeling, we provide a clearer picture of how E/I balance is preserved and where it fails in living neuronal networks.
Keywords: E/I balance; bursting; network dynamics; neuronal network.
Conflict of interest statement
The authors declare no competing interest.
Figures
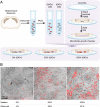
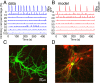
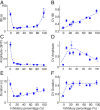

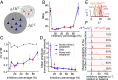

References
-
- Okun M., Lampl I., Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat. Neurosci. 11, 535–537 (2008). - PubMed
-
- Brunel N., Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons. J. Comput. Neurosci. 8, 183–208 (2000). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

