The intrinsic instability of the hydrolase domain of lipoprotein lipase facilitates its inactivation by ANGPTL4-catalyzed unfolding
- PMID: 33723082
- PMCID: PMC8000434
- DOI: 10.1073/pnas.2026650118
The intrinsic instability of the hydrolase domain of lipoprotein lipase facilitates its inactivation by ANGPTL4-catalyzed unfolding
Abstract
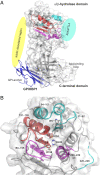
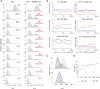
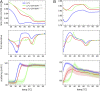
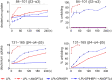
The complex between lipoprotein lipase (LPL) and its endothelial receptor (GPIHBP1) is responsible for the lipolytic processing of triglyceride-rich lipoproteins (TRLs) along the capillary lumen, a physiologic process that releases lipid nutrients for vital organs such as heart and skeletal muscle. LPL activity is regulated in a tissue-specific manner by endogenous inhibitors (angiopoietin-like [ANGPTL] proteins 3, 4, and 8), but the molecular mechanisms are incompletely understood. ANGPTL4 catalyzes the inactivation of LPL monomers by triggering the irreversible unfolding of LPL's α/β-hydrolase domain. Here, we show that this unfolding is initiated by the binding of ANGPTL4 to sequences near LPL's catalytic site, including β2, β3-α3, and the lid. Using pulse-labeling hydrogen‒deuterium exchange mass spectrometry, we found that ANGPTL4 binding initiates conformational changes that are nucleated on β3-α3 and progress to β5 and β4-α4, ultimately leading to the irreversible unfolding of regions that form LPL's catalytic pocket. LPL unfolding is context dependent and varies with the thermal stability of LPL's α/β-hydrolase domain (Tm of 34.8 °C). GPIHBP1 binding dramatically increases LPL stability (Tm of 57.6 °C), while ANGPTL4 lowers the onset of LPL unfolding by ∼20 °C, both for LPL and LPL•GPIHBP1 complexes. These observations explain why the binding of GPIHBP1 to LPL retards the kinetics of ANGPTL4-mediated LPL inactivation at 37 °C but does not fully suppress inactivation. The allosteric mechanism by which ANGPTL4 catalyzes the irreversible unfolding and inactivation of LPL is an unprecedented pathway for regulating intravascular lipid metabolism.
Keywords: GPIHBP1; HDX-MS; hypertriglyceridemia; intravascular lipolysis; intrinsic disorder.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

