Plasma IL-6 levels following corticosteroid therapy as an indicator of ICU length of stay in critically ill COVID-19 patients
- PMID: 33723251
- PMCID: PMC7958587
- DOI: 10.1038/s41420-021-00429-9
Plasma IL-6 levels following corticosteroid therapy as an indicator of ICU length of stay in critically ill COVID-19 patients
Abstract
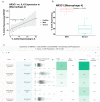
Intensive care unit (ICU) admissions and mortality in severe COVID-19 patients are driven by "cytokine storms" and acute respiratory distress syndrome (ARDS). Interim clinical trial results suggest that the corticosteroid dexamethasone displays better 28-day survival in severe COVID-19 patients requiring ventilation or oxygen. In this study, 10 out of 16 patients (62.5%) that had an average plasma IL-6 value over 10 pg/mL post administration of corticosteroids also had worse outcomes (i.e., ICU stay >15 days or death), compared to 8 out of 41 patients (19.5%) who did not receive corticosteroids (p-value = 0.0024). Given this potential association between post-corticosteroid IL-6 levels and COVID-19 severity, we hypothesized that the glucocorticoid receptor (GR or NR3C1) may be coupled to IL-6 expression in specific cell types that govern cytokine release syndrome (CRS). Examining single-cell RNA-seq data from BALF of severe COVID-19 patients and nearly 2 million cells from a pan-tissue scan shows that alveolar macrophages, smooth muscle cells, and endothelial cells co-express NR3C1 and IL-6, motivating future studies on the links between the regulation of NR3C1 function and IL-6 levels.
Conflict of interest statement
ADB is supported by Grants AI110173 and AI120698 from NIAID, 109593-62-RGRL from Amfar, and the HH Sheikh Khalifa Bin Zayed Al-Nahyan named professorship from Mayo Clinic. ADB is a consultant for Abbvie, is on scientific advisory boards for Nference and Zentalis, and is founder and President of Splissen therapeutics. WK reports grants from AstraZeneca, grants from Biogen, grants from Roche, outside the submitted work. One or more of the investigators from the Mayo Clinic associated with this project and Mayo Clinic have a Financial Conflict of Interest in the technology used in the research and that the investigator(s) and Mayo Clinic may stand to gain financially from the successful outcome of the research. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic Conflict of Interest policies. The authors on this manuscript from nference have equity in nference and have a financial interest in nference.
Figures







References
-
- COVID-19 Studies from the World Health Organization Database—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/who_table.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

