Wnt5A modulates integrin expression in a receptor-dependent manner in ovarian cancer cells
- PMID: 33723319
- PMCID: PMC7970989
- DOI: 10.1038/s41598-021-85356-6
Wnt5A modulates integrin expression in a receptor-dependent manner in ovarian cancer cells
Abstract
Wnt5A signals through various receptors that confer versatile biological functions. Here, we used Wnt5A overexpressing human ovarian SKOV-3 and OVCAR-3 stable clones for assessing integrin expression, cell proliferation, migration, invasion, and the ability of multicellular aggregates (MCAs) formation. We found here, that Wnt5A regulates differently the expression of its receptors in the stable Wnt5A overexpressing clones. The expression levels of Frizzled (FZD)-2 and -5, were increased in different clones. However ROR-1, -2 expression levels were differently regulated in clones. Wnt5A overexpressing clones showed increased cell proliferation, migration, and clonogenicity. Moreover, Wnt5A overexpressing SKOV-3 clone showed increased MCAs formation ability. Cell invasion had been increased in OVCAR-3-derived clones, while this was decreased in SKOV-3-derived clone. Importantly, αv integrin expression levels were increased in all assessed clones, accompanied by increased cell attachment to fibronectin and focal adhesion kinase activity. Moreover, the treatment of clones with Box5 as a Wnt5A/FZD5 antagonist abrogates ITGAV increase, cell proliferation, migration, and their attachment to fibronectin. Accordingly, we observed significantly higher expression levels of ITGAV and ITGB3 in human high-grade serous ovarian cancer specimens and ITGAV correlated positively with Wnt5A in metastatic serous type ovarian cancer. In summary, we hypothesize here, that Wnt5A/FZD-5 signaling modulate αv integrin expression levels that could be associated with ovarian cancer cell proliferation, migration, and fibronectin attachment.
Conflict of interest statement
The authors declare no competing interests.
Figures







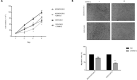
References
-
- Stewart B, Wild CP. World Cancer Report. IARC; 2014.
-
- Frankel A, Buckman R, Kerbel RS. Abrogation of taxol-induced G2-M arrest and apoptosis in human ovarian cancer cells grown as multicellular tumor spheroids. Cancer Res. 1997;57:2388–2393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

