Small compounds mimicking the adhesion molecule L1 improve recovery in a zebrafish demyelination model
- PMID: 33723325
- PMCID: PMC7960995
- DOI: 10.1038/s41598-021-85412-1
Small compounds mimicking the adhesion molecule L1 improve recovery in a zebrafish demyelination model
Abstract
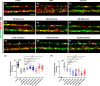
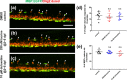
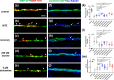
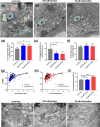
Demyelination leads to a loss of neurons, which results in, among other consequences, a severe reduction in locomotor function, and underlies several diseases in humans including multiple sclerosis and polyneuropathies. Considerable clinical progress has been made in counteracting demyelination. However, there remains a need for novel methods that reduce demyelination while concomitantly achieving remyelination, thus complementing the currently available tools to ameliorate demyelinating diseases. In this study, we used an established zebrafish demyelination model to test selected compounds, following a screening in cell culture experiments and in a mouse model of spinal cord injury that was aimed at identifying beneficial functions of the neural cell adhesion molecule L1. In comparison to mammalian nervous system disease models, the zebrafish allows testing of potentially promotive compounds more easily than what is possible in mammals. We found that our selected compounds tacrine and duloxetine significantly improved remyelination in the peripheral and central nervous system of transgenic zebrafish following pharmacologically induced demyelination. Given that both molecules are known to positively affect functions other than those related to L1 and in other disease contexts, we propose that their combined beneficial function raises hope for the use of these compounds in clinical settings.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

