Transcranial ultrafast ultrasound localization microscopy of brain vasculature in patients
- PMID: 33723412
- PMCID: PMC7610356
- DOI: 10.1038/s41551-021-00697-x
Transcranial ultrafast ultrasound localization microscopy of brain vasculature in patients
Abstract
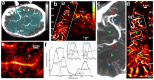
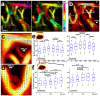
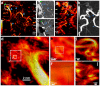
Changes in cerebral blood flow are associated with stroke, aneurysms, vascular cognitive impairment, neurodegenerative diseases and other pathologies. Brain angiograms, typically performed via computed tomography or magnetic resonance imaging, are limited to millimetre-scale resolution and are insensitive to blood-flow dynamics. Here we show that ultrafast ultrasound localization microscopy of intravenously injected microbubbles enables transcranial imaging of deep vasculature in the adult human brain at microscopic resolution and the quantification of haemodynamic parameters. Adaptive speckle tracking to correct for micrometric brain-motion artefacts and ultrasonic-wave aberrations induced during transcranial propagation allowed us to map the vascular network of tangled arteries to functionally characterize blood-flow dynamics at a resolution of up to 25 μm and to detect blood vortices in a small deep-seated aneurysm in a patient. Ultrafast ultrasound localization microscopy may facilitate the understanding of brain haemodynamics and of how vascular abnormalities in the brain are related to neurological pathologies.
Conflict of interest statement
M.P. and M.T. are co-founders and shareholders of Iconeus company commercializing ultrasound neuroimaging scanners. M.T. is co-inventor of the patent WO2012080614A1 filled on 2010-12-16 and licenced to Iconeus company. All other authors declare no competing interests.
Figures

Comment in
-
Device downsizing via signal enhancement.Nat Biomed Eng. 2021 Mar;5(3):195-196. doi: 10.1038/s41551-021-00708-x. Nat Biomed Eng. 2021. PMID: 33727712 No abstract available.
References
-
- Liesz A. The vascular side of Alzheimer’s disease. Science. 2019;365:223–224. - PubMed
-
- O’Brien JT, Thomas A. Vascular dementia. The Lancet. 2015;386:1698–1706. - PubMed
-
- Betzig E, et al. Imaging Intracellular Fluorescent Proteins at Nanometer Resolution. Science. 2006;313:1642–1645. - PubMed
-
- Errico C, et al. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature. 2015;527:499–502. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

