Divergent pallidal pathways underlying distinct Parkinsonian behavioral deficits
- PMID: 33723433
- PMCID: PMC8907079
- DOI: 10.1038/s41593-021-00810-y
Divergent pallidal pathways underlying distinct Parkinsonian behavioral deficits
Abstract
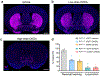
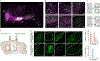
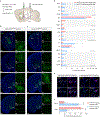
The basal ganglia regulate a wide range of behaviors, including motor control and cognitive functions, and are profoundly affected in Parkinson's disease (PD). However, the functional organization of different basal ganglia nuclei has not been fully elucidated at the circuit level. In this study, we investigated the functional roles of distinct parvalbumin-expressing neuronal populations in the external globus pallidus (GPe-PV) and their contributions to different PD-related behaviors. We demonstrate that substantia nigra pars reticulata (SNr)-projecting GPe-PV neurons and parafascicular thalamus (PF)-projecting GPe-PV neurons are associated with locomotion and reversal learning, respectively. In a mouse model of PD, we found that selective manipulation of the SNr-projecting GPe-PV neurons alleviated locomotor deficit, whereas manipulation of the PF-projecting GPe-PV neurons rescued the impaired reversal learning. Our findings establish the behavioral importance of two distinct GPe-PV neuronal populations and, thereby, provide a new framework for understanding the circuit basis of different behavioral deficits in the Parkinsonian state.
Conflict of interest statement
Competing interests
K.C. is a co-inventor on a patent application owned by MIT covering the SHIELD technology and is a co-founder of LifeCanvas Technologies.
Figures






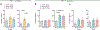



Comment in
-
Distinct globus pallidus circuits regulate motor and cognitive functions.Trends Neurosci. 2021 Aug;44(8):597-599. doi: 10.1016/j.tins.2021.06.001. Epub 2021 Jun 15. Trends Neurosci. 2021. PMID: 34144845 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

