Infectious Period of Severe Acute Respiratory Syndrome Coronavirus 2 in 17 Nursing Home Residents-Arkansas, June-August 2020
- PMID: 33723510
- PMCID: PMC7928697
- DOI: 10.1093/ofid/ofab048
Infectious Period of Severe Acute Respiratory Syndrome Coronavirus 2 in 17 Nursing Home Residents-Arkansas, June-August 2020
Abstract
Background: To estimate the infectious period of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in older adults with underlying conditions, we assessed duration of coronavirus disease 2019 (COVID-19) symptoms, reverse-transcription polymerase chain reaction (RT-PCR) positivity, and culture positivity among nursing home residents.
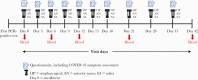
Methods: We enrolled residents within 15 days of their first positive SARS-CoV-2 test (diagnosis) at an Arkansas facility from July 7 to 15, 2020 and instead them for 42 days. Every 3 days for 21 days and then weekly, we assessed COVID-19 symptoms, collected specimens (oropharyngeal, anterior nares, and saliva), and reviewed medical charts. Blood for serology was collected on days 0, 6, 12, 21, and 42. Infectivity was defined by positive culture. Duration of culture positivity was compared with duration of COVID-19 symptoms and RT-PCR positivity. Data were summarized using measures of central tendency, frequencies, and proportions.
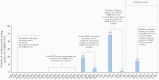
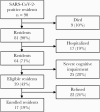
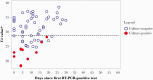
Results: We enrolled 17 of 39 (44%) eligible residents. Median participant age was 82 years (range, 58-97 years). All had ≥3 underlying conditions. Median duration of RT-PCR positivity was 22 days (interquartile range [IQR], 8-31 days) from diagnosis; median duration of symptoms was 42 days (IQR, 28-49 days). Of 9 (53%) participants with any culture-positive specimens, 1 (11%) severely immunocompromised participant remained culture-positive 19 days from diagnosis; 8 of 9 (89%) were culture-positive ≤8 days from diagnosis. Seroconversion occurred in 12 of 12 (100%) surviving participants with ≥1 blood specimen; all participants were culture-negative before seroconversion.
Conclusions: Duration of infectivity was considerably shorter than duration of symptoms and RT-PCR positivity. Severe immunocompromise may prolong SARS-CoV-2 infectivity. Seroconversion indicated noninfectivity in this cohort.
Keywords: COVID-19; RT-PCR; SARS-CoV-2; infectivity; nursing homes.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2021.
Figures

References
-
- Centers for Disease Control and Prevention. CDC COVID Data tracker. Available at: https://covid.cdc.gov/covid-data-tracker/#cases_totalcases. Accessed 13 September 2020.
-
- Centers for Medicare and Medicaid Services. COVID-19 nursing home data. Available at: https://data.cms.gov/stories/s/COVID-19-Nursing-Home-Data/bkwz-xpvg/. Accessed 13 September 2020.
-
- Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581:465–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

