New aspects of endocrine control of atrial fibrillation and possibilities for clinical translation
- PMID: 33723575
- PMCID: PMC8208746
- DOI: 10.1093/cvr/cvab080
New aspects of endocrine control of atrial fibrillation and possibilities for clinical translation
Abstract
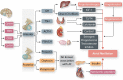
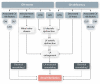
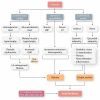
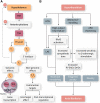
Hormones are potent endo-, para-, and autocrine endogenous regulators of the function of multiple organs, including the heart. Endocrine dysfunction promotes a number of cardiovascular diseases, including atrial fibrillation (AF). While the heart is a target for endocrine regulation, it is also an active endocrine organ itself, secreting a number of important bioactive hormones that convey significant endocrine effects, but also through para-/autocrine actions, actively participate in cardiac self-regulation. The hormones regulating heart-function work in concert to support myocardial performance. AF is a serious clinical problem associated with increased morbidity and mortality, mainly due to stroke and heart failure. Current therapies for AF remain inadequate. AF is characterized by altered atrial function and structure, including electrical and profibrotic remodelling in the atria and ventricles, which facilitates AF progression and hampers its treatment. Although features of this remodelling are well-established and its mechanisms are partly understood, important pathways pertinent to AF arrhythmogenesis are still unidentified. The discovery of these missing pathways has the potential to lead to therapeutic breakthroughs. Endocrine dysfunction is well-recognized to lead to AF. In this review, we discuss endocrine and cardiocrine signalling systems that directly, or as a consequence of an underlying cardiac pathology, contribute to AF pathogenesis. More specifically, we consider the roles of products from the hypothalamic-pituitary axis, the adrenal glands, adipose tissue, the renin-angiotensin system, atrial cardiomyocytes, and the thyroid gland in controlling atrial electrical and structural properties. The influence of endocrine/paracrine dysfunction on AF risk and mechanisms is evaluated and discussed. We focus on the most recent findings and reflect on the potential of translating them into clinical application.
Keywords: Arrhythmia; Atrial fibrillation; Endocrine system; Heart.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2021. For permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Carpenter PC. Diagnostic evaluation of Cushing's syndrome. Endocrinol Metab Clin North Am 1988;17:445–472. - PubMed
-
- van der Hooft CS, Heeringa J, Brusselle GG, Hofman A, Witteman JC, Kingma JH, Sturkenboom MC, Stricker BH. Corticosteroids and the risk of atrial fibrillation. Arch Intern Med 2006;166:1016–1020. - PubMed
-
- Di Dalmazi G, Vicennati V, Pizzi C, Mosconi C, Tucci L, Balacchi C, Cosentino ER, Paolisso P, Fanelli F, Gambineri A, Pelusi C, Repaci A, Garelli S, Galie N, Borghi C, Golfieri R, Pagotto U. Prevalence and incidence of atrial fibrillation in a large cohort of adrenal incidentalomas: a long-term study. J Clin Endocrinol Metab 2020;105. - PubMed
-
- Neary NM, Booker OJ, Abel BS, Matta JR, Muldoon N, Sinaii N, Pettigrew RI, Nieman LK, Gharib AM. Hypercortisolism is associated with increased coronary arterial atherosclerosis: analysis of noninvasive coronary angiography using multidetector computerized tomography. J Clin Endocrinol Metab 2013;98:2045–2052. - PMC - PubMed
-
- Mancini T, Kola B, Mantero F, Boscaro M, Arnaldi G. High cardiovascular risk in patients with Cushing's syndrome according to 1999 WHO/ISH guidelines. Clin Endocrinol 2004;61:768–777. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

