Role of Pelvic Lymph Node Resection in Vulvar Squamous Cell Cancer: A Subset Analysis of the AGO-CaRE-1 Study
- PMID: 33723714
- PMCID: PMC8460538
- DOI: 10.1245/s10434-021-09744-y
Role of Pelvic Lymph Node Resection in Vulvar Squamous Cell Cancer: A Subset Analysis of the AGO-CaRE-1 Study
Abstract
Background: As the population at risk for pelvic nodal involvement remains poorly described, the role of pelvic lymphadenectomy (LAE) in vulvar squamous cell cancer (VSCC) has been a matter of discussion for decades.
Methods: In the AGO-CaRE-1 study, 1618 patients with International Federation of Gynecology and Obstetrics (FIGO) stage IB or higher primary VSCC treated at 29 centers in Germany between 1998 and 2008 were documented. In this analysis, only patients with pelvic LAE (n = 70) were analyzed with regard to prognosis and correlation between inguinal and pelvic lymph node involvement.
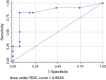
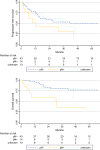
Results: The majority of patients had T1b/T2 tumors (n = 47; 67.1%), with a median diameter of 40 mm (2-240 mm); 54/70 patients (77.1%) who received pelvic LAE had positive groin nodes. For 42 of these 54 patients, the number of affected groin nodes had been documented as a median of 3; 14/42 (33.3%) of these patients had histologically confirmed pelvic nodal metastases (median number of affected pelvic nodes 3 [1-12]). In these 14 patients, the median number of affected groin nodes was 7 (1-30), with a groin metastases median maximum diameter of 42.5 mm (12-50). Receiver operating characteristic analysis showed an area under the curve of 0.85, with 83.3% sensitivity and 92.6% specificity for the prediction of pelvic involvement in cases of six or more positive groin nodes. No cases of pelvic nodal involvement without groin metastases were observed. Prognosis in cases of pelvic metastasis was poor, with a median progression-free survival of only 12.5 months.
Conclusion: For the majority of node-positive patients with VSCC, pelvic nodal staging appears unnecessary since a relevant risk for pelvic nodal involvement only seems to be present in highly node-positive disease.
© 2021. The Author(s).
Figures
Similar articles
-
Pelvic lymphadenectomy in vulvar cancer and its impact on prognosis and outcome.Arch Gynecol Obstet. 2022 Jan;305(1):233-240. doi: 10.1007/s00404-021-06156-x. Epub 2021 Aug 13. Arch Gynecol Obstet. 2022. PMID: 34387725 Free PMC article.
-
Number of Nodes Removed With Inguinofemoral Lymphadenectomy and Risk of Isolated Groin Recurrence in Women With FIGO Stage IB-II Squamous Cell Vulvar Cancer.Int J Gynecol Cancer. 2018 Oct;28(8):1600-1605. doi: 10.1097/IGC.0000000000001326. Int J Gynecol Cancer. 2018. PMID: 30036227
-
The role of preoperative computerized tomography (CT) scan of the pelvis and groin in the management of clinically early staged vulva squamous cell carcinoma.Gynecol Oncol. 2020 May;157(2):444-449. doi: 10.1016/j.ygyno.2020.01.031. Epub 2020 Jan 24. Gynecol Oncol. 2020. PMID: 31987600
-
Staging for vulvar cancer.Best Pract Res Clin Obstet Gynaecol. 2015 Aug;29(6):802-11. doi: 10.1016/j.bpobgyn.2015.01.004. Epub 2015 Mar 4. Best Pract Res Clin Obstet Gynaecol. 2015. PMID: 25842047 Review.
-
Prognostic role of inguinal lymphadenectomy in vulvar squamous carcinoma: younger and older patients should be equally treated. A prospective study and literature review.Gynecol Oncol. 2015 Jun;137(3):373-9. doi: 10.1016/j.ygyno.2015.03.013. Epub 2015 Apr 15. Gynecol Oncol. 2015. PMID: 25887098 Review.
Cited by
-
Pelvic Lymphadenectomy in Vulvar Cancer - Does it make sense?Geburtshilfe Frauenheilkd. 2020 Dec;80(12):1221-1228. doi: 10.1055/a-1120-0138. Epub 2020 Dec 3. Geburtshilfe Frauenheilkd. 2020. PMID: 33293730 Free PMC article.
-
Risk for Pelvic Metastasis and Role of Pelvic Lymphadenectomy in Node-Positive Vulvar Cancer-Results from the AGO-VOP.2 QS Vulva Study.Cancers (Basel). 2022 Jan 14;14(2):418. doi: 10.3390/cancers14020418. Cancers (Basel). 2022. PMID: 35053582 Free PMC article.
References
-
- Homesley HD, Bundy BN, Sedlis A, Adcock L. Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstet Gynecol. 1986;68(6):733–740. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials