Dimethyl sulfoxide stimulates the AhR-Jdp2 axis to control ROS accumulation in mouse embryonic fibroblasts
- PMID: 33723743
- PMCID: PMC8986748
- DOI: 10.1007/s10565-021-09592-2
Dimethyl sulfoxide stimulates the AhR-Jdp2 axis to control ROS accumulation in mouse embryonic fibroblasts
Abstract
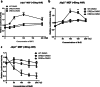
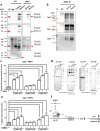
The aryl hydrocarbon receptor (AhR) is a ligand-binding protein that responds to environmental aromatic hydrocarbons and stimulates the transcription of downstream phase I enzyme-related genes by binding the cis element of dioxin-responsive elements (DREs)/xenobiotic-responsive elements. Dimethyl sulfoxide (DMSO) is a well-known organic solvent that is often used to dissolve phase I reagents in toxicology and oxidative stress research experiments. In the current study, we discovered that 0.1% DMSO significantly induced the activation of the AhR promoter via DREs and produced reactive oxygen species, which induced apoptosis in mouse embryonic fibroblasts (MEFs). Moreover, Jun dimerization protein 2 (Jdp2) was found to be required for activation of the AhR promoter in response to DMSO. Coimmunoprecipitation and chromatin immunoprecipitation studies demonstrated that the phase I-dependent transcription factors, AhR and the AhR nuclear translocator, and phase II-dependent transcription factors such as nuclear factor (erythroid-derived 2)-like 2 (Nrf2) integrated into DRE sites together with Jdp2 to form an activation complex to increase AhR promoter activity in response to DMSO in MEFs. Our findings provide evidence for the functional role of Jdp2 in controlling the AhR gene via Nrf2 and provide insights into how Jdp2 contributes to the regulation of ROS production and the cell spreading and apoptosis produced by the ligand DMSO in MEFs.
Keywords: AhR; Dimethyl sulfoxide; Jun dimerization protein 2; Mouse embryonic fibroblasts; Nrf2; Reactive oxygen species.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

