The DNA methylation inhibitor RG108 protects against noise-induced hearing loss
- PMID: 33723744
- PMCID: PMC8490244
- DOI: 10.1007/s10565-021-09596-y
The DNA methylation inhibitor RG108 protects against noise-induced hearing loss
Abstract
Background: Noise-induced hearing loss represents a commonly diagnosed type of hearing disability, severely impacting the quality of life of individuals. The current work is aimed at assessing the effects of DNA methylation on noise-induced hearing loss.
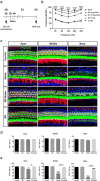
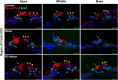
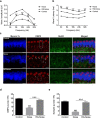
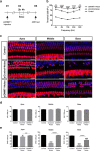
Methods: Blocking DNA methyltransferase 1 (DNMT1) activity with a selective inhibitor RG108 or silencing DNMT1 with siRNA was used in this study. Auditory brainstem responses were measured at baseline and 2 days after trauma in mice to assess auditory functions. Whole-mount immunofluorescent staining and confocal microcopy of mouse inner ear specimens were performed to analyze noise-induced damage in cochleae and the auditory nerve at 2 days after noise exposure.
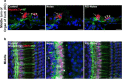
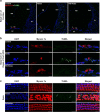
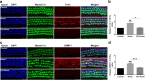
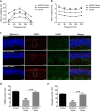
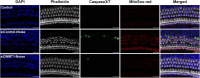
Results: The results showed that noise exposure caused threshold elevation of auditory brainstem responses and cochlear hair cell loss. Whole-mount cochlea staining revealed a reduction in the density of auditory ribbon synapses between inner hair cells and spiral ganglion neurons. Inhibition of DNA methyltransferase activity via a non-nucleoside specific pharmacological inhibitor, RG108, or silencing of DNA methyltransferase-1 with siRNA significantly attenuated ABR threshold elevation, hair cell damage, and the loss of auditory synapses.
Conclusions: This study suggests that inhibition of DNMT1 ameliorates noise-induced hearing loss and indicates that DNMT1 may be a promising therapeutic target. Graphical Headlights • RG108 protected against noise-induced hearing loss • RG108 administration protected against noise-induced hair cell loss and auditory neural damage. • RG108 administration attenuated oxidative stress-induced DNA damage and subsequent apoptosis-mediated cell loss in the cochlea after noise exposure.
Keywords: Cochlea; DNA methylation; Hair cells; Noise-induced hearing loss.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

