Craniofacial Development: Neural Crest in Molecular Embryology
- PMID: 33723764
- PMCID: PMC8010074
- DOI: 10.1007/s12105-021-01301-z
Craniofacial Development: Neural Crest in Molecular Embryology
Abstract
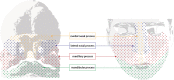
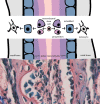
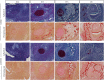
Craniofacial development, one of the most complex sequences of developmental events in embryology, features a uniquely transient, pluripotent stem cell-like population known as the neural crest (NC). Neural crest cells (NCCs) originate from the dorsal aspect of the neural tube and migrate along pre-determined routes into the developing branchial arches and frontonasal plate. The exceptional rates of proliferation and migration of NCCs enable their diverse contribution to a wide variety of craniofacial structures. Subsequent differentiation of these cells gives rise to cartilage, bones, and a number of mesenchymally-derived tissues. Deficiencies in any stage of differentiation can result in facial clefts and abnormalities associated with craniofacial syndromes. A small number of conserved signaling pathways are involved in controlling NC differentiation and craniofacial development. They are used in a reiterated fashion to help define precise temporospatial cell and tissue formation. Although many aspects of their cellular and molecular control have yet to be described, it is clear that together they form intricately integrated signaling networks required for spatial orientation and developmental stability and plasticity, which are hallmarks of craniofacial development. Mutations that affect the functions of these signaling pathways are often directly or indirectly identified in congenital syndromes. Clinical applications of NC-derived mesenchymal stem/progenitor cells, persistent into adulthood, hold great promise for tissue repair and regeneration. Realization of NCC potential for regenerative therapies motivates understanding of the intricacies of cell communication and differentiation that underlie the complexities of NC-derived tissues.
Keywords: Bone; Cartilage; Neural crest; Orofacial development; Signalling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Saint-Jeannet J-P, editor. Neural Crest Induction and Differentiation [Internet]. Springer US; 2006 [cited 2020 Oct 26]. https://www.springer.com/gp/book/9780387351360
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

