Side-effects of carbetocin to prevent postpartum hemorrhage: A systematic review and meta-analysis of randomized controlled trials
- PMID: 33723868
- PMCID: PMC7961157
- DOI: 10.1002/prp2.745
Side-effects of carbetocin to prevent postpartum hemorrhage: A systematic review and meta-analysis of randomized controlled trials
Abstract
Postpartum hemorrhage (PPH) increases the risk of maternal death worldwide. Heat-stable carbetocin, a long-acting oxytocin analog, is a newer uterotonic agent. Clinicians do not fully understand its side-effects, particularly the unanticipated side-effects. The aim of this study is to investigate the side-effects of carbetocin to PPH. The Cochrane Library, Web of Science, PubMed, Elsevier ScienceDirect, Embase, and ClinicalTrials.gov were searched from the inception to September 2020. Randomized controlled trials (RCTs) that considered pregnant women who received carbetocin before delivery and provided at least one adverse event were included. Statistical analysis included random or fixed-effect meta-analyses using relative risk. Stratified analyses and sensitivity analyses were also performed. Begger's and Egger's test and funnel plots were used to assess the publication bias. Seventeen RCTs involving 32,702 women were included, and all these studies ranked as medium- to high-quality. Twenty-four side-effects were reported. The use of carbetocin had a lower risk of vomiting in intravenously (0.53, 0.30 to 0.93) and cesarean birth (0.51, 0.32 to 0.81) women, and had a slightly higher risk of diarrhea (8.00, 1.02 to 62.79) compared with oxytocin intervention. No significant difference was found among other side-effects. Evidence from our systematic review and meta-analysis of 17 RCTs suggested that the risk of vomiting decreased with carbetocin use in the prevention of PPH after delivery.
Keywords: carbetocin; meta-analysis; postpartum hemorrhage; side-effects; systematic review.
© 2021 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
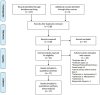
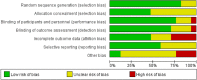
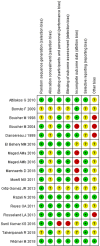
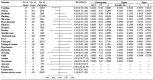
Similar articles
-
Uterotonic drugs to prevent postpartum haemorrhage: a network meta-analysis.Health Technol Assess. 2019 Feb;23(9):1-356. doi: 10.3310/hta23090. Health Technol Assess. 2019. PMID: 30821683 Free PMC article.
-
Efficacy of carbetocin in the prevention of postpartum hemorrhage: a systematic review and Bayesian meta-analysis of randomized trials.J Matern Fetal Neonatal Med. 2021 Jul;34(14):2303-2316. doi: 10.1080/14767058.2019.1664463. Epub 2019 Sep 19. J Matern Fetal Neonatal Med. 2021. PMID: 31537134
-
The effect of carbetocin compared to misoprostol in management of the third stage of labor and prevention of postpartum hemorrhage: a systematic review.Syst Rev. 2018 Oct 20;7(1):170. doi: 10.1186/s13643-018-0832-4. Syst Rev. 2018. PMID: 30342555 Free PMC article.
-
Carbetocin versus oxytocin for the prevention of postpartum hemorrhage: A meta-analysis of randomized controlled trials in cesarean deliveries.Taiwan J Obstet Gynecol. 2018 Jun;57(3):332-339. doi: 10.1016/j.tjog.2018.04.002. Taiwan J Obstet Gynecol. 2018. PMID: 29880160
-
Utilization of carbetocin for prevention of postpartum hemorrhage after cesarean section: a randomized clinical trial.Arch Gynecol Obstet. 2009 Nov;280(5):707-12. doi: 10.1007/s00404-009-0973-8. Epub 2009 Feb 20. Arch Gynecol Obstet. 2009. PMID: 19229549 Clinical Trial.
Cited by
-
Peripherally restricted oxytocin is sufficient to reduce food intake and motivation, while CNS entry is required for locomotor and taste avoidance effects.Diabetes Obes Metab. 2023 Mar;25(3):856-877. doi: 10.1111/dom.14937. Epub 2023 Jan 9. Diabetes Obes Metab. 2023. PMID: 36495318 Free PMC article.
-
Risk Factors for Carbetocin Failure after a Cesarean Section: Is Obesity One of Them?J Clin Med. 2021 Aug 24;10(17):3767. doi: 10.3390/jcm10173767. J Clin Med. 2021. PMID: 34501215 Free PMC article.
-
Heat-Stable Carbetocin in the Management of Postpartum Haemorrhage in Low- and Middle-Income Countries: A Comprehensive Review of Clinical Evidence, Cost-Effectiveness, Implementation Challenges and Adoption Strategies.Int J Womens Health. 2025 Jun 3;17:1615-1630. doi: 10.2147/IJWH.S515252. eCollection 2025. Int J Womens Health. 2025. PMID: 40487679 Free PMC article. Review.
-
Side-effects of intravenously versus intramuscularly oxytocin for postpartum hemorrhage: a systematic review and meta-analysis of randomized controlled trials.Front Pharmacol. 2023 Dec 22;14:1273771. doi: 10.3389/fphar.2023.1273771. eCollection 2023. Front Pharmacol. 2023. PMID: 38186656 Free PMC article.
-
Case report: A relatively rare adverse event caused by carbetocin- placenta interception.Front Pharmacol. 2023 Mar 23;14:1112694. doi: 10.3389/fphar.2023.1112694. eCollection 2023. Front Pharmacol. 2023. PMID: 37033610 Free PMC article.
References
-
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. The Lancet Global Health. 2014;2(6):e323‐e333. - PubMed
-
- WHO recommendations: Uterotonics for the prevention of postpartum haemorrhage. Geneva; 2018. - PubMed
-
- WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Geneva; 2012. - PubMed
-
- Torloni MR, Gomes Freitas C, Kartoglu UH, Metin Gulmezoglu A, Widmer M. Quality of oxytocin available in low‐ and middle‐income countries: a systematic review of the literature. BJOG: an international journal of obstetrics and gynaecology. 2016;123(13):2076‐2086. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

