Plasmodesmata play pivotal role in sucrose supply to Meloidogyne graminicola-caused giant cells in rice
- PMID: 33723908
- PMCID: PMC8035636
- DOI: 10.1111/mpp.13042
Plasmodesmata play pivotal role in sucrose supply to Meloidogyne graminicola-caused giant cells in rice
Abstract
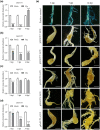
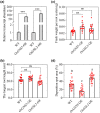
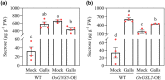
On infection, plant-parasitic nematodes establish feeding sites in roots from which they take up carbohydrates among other nutrients. Knowledge on how carbohydrates are supplied to the nematodes' feeding sites is limited. Here, gene expression analyses showed that RNA levels of OsSWEET11 to OsSWEET15 were extremely low in both Meloidogyne graminicola (Mg)-caused galls and noninoculated roots. All the rice sucrose transporter genes, OsSUT1 to OsSUT5, were either down-regulated in Mg-caused galls compared with noninoculated rice roots or had very low transcript abundance. OsSUT1 was the only gene up-regulated in galls, at 14 days postinoculation (dpi), after being highly down-regulated at 3 and 7 dpi. OsSUT4 was down-regulated at 3 dpi. No noticeable OsSUTs promoter activities were detected in Mg-caused galls of pOsSUT1 to -5::GUS rice lines. Loading experiments with carboxyfluorescein diacetate (CFDA) demonstrated that symplastic connections exist between phloem and Mg-caused giant cells (GCs). According to data from OsGNS5- and OsGSL2-overexpressing rice plants that had decreased and increased callose deposition, respectively, callose negatively affected Mg parasitism and sucrose supply to Mg-caused GCs. Our results suggest that plasmodesmata-mediated sucrose transport plays a pivotal role in sucrose supply from rice root phloem to Mg-caused GCs, and OsSWEET11 to -15 and OsSUTs are not major players in it, although further functional analysis is needed for OsSUT1 and OsSUT4.
Keywords: Meloidogyne graminicola; OsSUT s; callose deposition; giant cells; plasmodesmata; sucrose transport.
© 2021 The Authors. Molecular Plant Pathology published by British Society for Plant Pathology and John Wiley & Sons Ltd.
Figures


Similar articles
-
Phloem loading in rice leaves depends strongly on the apoplastic pathway.J Exp Bot. 2021 May 4;72(10):3723-3738. doi: 10.1093/jxb/erab085. J Exp Bot. 2021. PMID: 33624763
-
Proteome-Wide Analyses Provide New Insights into the Compatible Interaction of Rice with the Root-Knot Nematode Meloidogyne graminicola.Int J Mol Sci. 2020 Aug 6;21(16):5640. doi: 10.3390/ijms21165640. Int J Mol Sci. 2020. PMID: 32781661 Free PMC article.
-
Transcriptomic and histological responses of African rice (Oryza glaberrima) to Meloidogyne graminicola provide new insights into root-knot nematode resistance in monocots.Ann Bot. 2017 Mar 1;119(5):885-899. doi: 10.1093/aob/mcw256. Ann Bot. 2017. PMID: 28334204 Free PMC article.
-
The mechanism of phloem loading in rice (Oryza sativa).Mol Cells. 2012 May;33(5):431-8. doi: 10.1007/s10059-012-0071-9. Epub 2012 Mar 26. Mol Cells. 2012. PMID: 22453778 Free PMC article. Review.
-
Sucrose transporter in rice.Plant Signal Behav. 2021 Nov 2;16(11):1952373. doi: 10.1080/15592324.2021.1952373. Epub 2021 Jul 16. Plant Signal Behav. 2021. PMID: 34269147 Free PMC article. Review.
Cited by
-
AtSWEET1 negatively regulates plant susceptibility to root-knot nematode disease.Front Plant Sci. 2023 Feb 7;14:1010348. doi: 10.3389/fpls.2023.1010348. eCollection 2023. Front Plant Sci. 2023. PMID: 36824200 Free PMC article.
-
From Raffinose Family Oligosaccharides to Sucrose and Hexoses: Gene Expression Profiles Underlying Host-to-Nematode Carbon Delivery in Cucumis sativus Roots.Front Plant Sci. 2022 Feb 17;13:823382. doi: 10.3389/fpls.2022.823382. eCollection 2022. Front Plant Sci. 2022. PMID: 35251093 Free PMC article.
-
Rice SUT and SWEET Transporters.Int J Mol Sci. 2021 Oct 18;22(20):11198. doi: 10.3390/ijms222011198. Int J Mol Sci. 2021. PMID: 34681858 Free PMC article. Review.
-
Morphological characterization reveals new insights into giant cell development of Meloidogyne graminicola on rice.Planta. 2022 Feb 19;255(3):70. doi: 10.1007/s00425-022-03852-z. Planta. 2022. PMID: 35184234 Free PMC article.
-
Analysis of Soluble Sugar Content in Minute Quantities of Rice Tissues by GC-MS.Bio Protoc. 2021 Jul 5;11(13):e4077. doi: 10.21769/BioProtoc.4077. eCollection 2021 Jul 5. Bio Protoc. 2021. PMID: 34327274 Free PMC article.
References
-
- Banora, M.Y. , Rodiuc, N. , Baldacci‐Cresp, F. , Smertenko, A. , Bleve‐Zacheo, T. , Mellilo, M.T. et al. (2011) Feeding cells induced by phytoparasitic nematodes require γ‐tubulin ring complex for microtubule reorganization. PLoS Pathogens, 7, e1002343.–10.1371/journal.ppat.1002343 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

