CT-derived Chest Muscle Metrics for Outcome Prediction in Patients with COVID-19
- PMID: 33724065
- PMCID: PMC7971428
- DOI: 10.1148/radiol.2021204141
CT-derived Chest Muscle Metrics for Outcome Prediction in Patients with COVID-19
Abstract
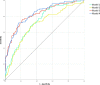
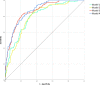
Background Lower muscle mass is a known predictor of unfavorable outcomes, but its prognostic impact on patients with COVID-19 is unknown. Purpose To investigate the contribution of CT-derived muscle status in predicting clinical outcomes in patients with COVID-19. Materials and Methods Clinical or laboratory data and outcomes (intensive care unit [ICU] admission and death) were retrospectively retrieved for patients with reverse transcriptase polymerase chain reaction-confirmed SARS-CoV-2 infection, who underwent chest CT on admission in four hospitals in Northern Italy from February 21 to April 30, 2020. The extent and type of pulmonary involvement, mediastinal lymphadenopathy, and pleural effusion were assessed. Cross-sectional areas and attenuation by paravertebral muscles were measured on axial CT images at the T5 and T12 vertebral level. Multivariable linear and binary logistic regression, including calculation of odds ratios (ORs) with 95% CIs, were used to build four models to predict ICU admission and death, which were tested and compared by using receiver operating characteristic curve analysis. Results A total of 552 patients (364 men and 188 women; median age, 65 years [interquartile range, 54-75 years]) were included. In a CT-based model, lower-than-median T5 paravertebral muscle areas showed the highest ORs for ICU admission (OR, 4.8; 95% CI: 2.7, 8.5; P < .001) and death (OR, 2.3; 95% CI: 1.0, 2.9; P = .03). When clinical variables were included in the model, lower-than-median T5 paravertebral muscle areas still showed the highest ORs for both ICU admission (OR, 4.3; 95%: CI: 2.5, 7.7; P < .001) and death (OR, 2.3; 95% CI: 1.3, 3.7; P = .001). At receiver operating characteristic analysis, the CT-based model and the model including clinical variables showed the same area under the receiver operating characteristic curve (AUC) for ICU admission prediction (AUC, 0.83; P = .38) and were not different in terms of predicting death (AUC, 0.86 vs AUC, 0.87, respectively; P = .28). Conclusion In hospitalized patients with COVID-19, lower muscle mass on CT images was independently associated with intensive care unit admission and in-hospital mortality. © RSNA, 2021 Online supplemental material is available for this article.
Figures


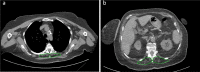
References
-
- Guo T, Shen Q, Guo W, et al. Clinical Characteristics of Elderly Patients with COVID-19 in Hunan Province, China: A Multicenter, Retrospective Study. Gerontology 2020;467–475. doi: 10.1159/000508734 - PubMed
-
- Albano D, Messina C, Vitale J, Sconfienza LM. Imaging of sarcopenia: old evidence and new insights. Eur Radiol 2020;2199–2208. doi: 10.1007/s00330-019-06573-2 - PubMed
-
- Boutin RD, Katz JR, Chaudhari AJ, et al. Association of adipose tissue and skeletal muscle metrics with overall survival and postoperative complications in soft tissue sarcoma patients: an opportunistic study using computed tomography. Quant Imaging Med Surg 2020;1580–1589. doi: 10.21037/qims.2020.02.09 - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

