Pathogenicity and virulence of Japanese encephalitis virus: Neuroinflammation and neuronal cell damage
- PMID: 33724154
- PMCID: PMC7971234
- DOI: 10.1080/21505594.2021.1899674
Pathogenicity and virulence of Japanese encephalitis virus: Neuroinflammation and neuronal cell damage
Abstract
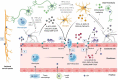
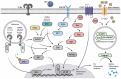
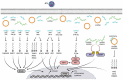
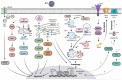
Thousands of human deaths occur annually due to Japanese encephalitis (JE), caused by Japanese encephalitis virus. During the virus infection of the central nervous system, reactive gliosis, uncontrolled inflammatory response, and neuronal cell death are considered as the characteristic features of JE. To date, no specific treatment has been approved to overcome JE, indicating a need for the development of novel therapies. In this article, we focused on basic biological mechanisms in glial (microglia and astrocytes) and neuronal cells that contribute to the onset of neuroinflammation and neuronal cell damage during Japanese encephalitis virus infection. We also provided comprehensive knowledge about anti-JE therapies tested in clinical or pre-clinical settings, and discussed recent therapeutic strategies that could be employed for JE treatment. The improved understanding of JE pathogenesis might lay a foundation for the development of novel therapies to halt JE.Abbreviations AKT: a serine/threonine-specific protein kinase; AP1: activator protein 1; ASC: apoptosis-associated speck-like protein containing a CARD; ASK1: apoptosis signal-regulated kinase 1; ATF3/4/6: activating transcription factor 3/4/6; ATG5/7: autophagy-related 5/7; BBB: blood-brain barrier; Bcl-3/6: B-cell lymphoma 3/6 protein; CCL: C-C motif chemokine ligand; CCR2: C-C motif chemokine receptor 2; CHOP: C/EBP homologous protein; circRNA: circular RNA; CNS: central nervous system; CXCL: C-X-C motif chemokine ligand; dsRNA: double-stranded RNA; EDEM1: endoplasmic reticulum degradation enhancer mannosidase alpha-like 1; eIF2-ɑ: eukaryotic initiation factor 2 alpha; ER: endoplasmic reticulum; ERK: extracellular signal-regulated kinase; GRP78: 78-kDa glucose-regulated protein; ICAM: intercellular adhesion molecule; IFN: interferon; IL: interleukin; iNOS: inducible nitric oxide synthase; IRAK1/2: interleukin-1 receptor-associated kinase 1/2; IRE-1: inositol-requiring enzyme 1; IRF: interferon regulatory factor; ISG15: interferon-stimulated gene 15; JE: Japanese encephalitis; JEV: Japanese encephalitis virus; JNK: c-Jun N-terminal kinase; LAMP2: lysosome-associated membrane protein type 2; LC3-I/II: microtubule-associated protein 1 light chain 3-I/II; lncRNA: long non-coding RNA; MAPK: mitogen-activated protein kinase; miR/miRNA: microRNA; MK2: mitogen-activated protein kinase-activated protein kinase 2; MKK4: mitogen-activated protein kinase kinase 4; MLKL: mixed-linage kinase domain-like protein; MMP: matrix metalloproteinase; MyD88: myeloid differentiation factor 88; Nedd4: neural precursor cell-expressed developmentally downregulated 4; NF-κB: nuclear factor kappa B; NKRF: nuclear factor kappa B repressing factor; NLRP3: NLR family pyrin domain containing 3; NMDAR: N-methyl-D-aspartate receptor; NO: nitric oxide; NS2B/3/4: JEV non-structural protein 2B/3/4; P: phosphorylation. p38: mitogen-activated protein kinase p38; PKA: protein kinase A; PAK4: p21-activated kinase 4; PDFGR: platelet-derived growth factor receptor; PERK: protein kinase R-like endoplasmic reticulum kinase; PI3K: phosphoinositide 3-kinase; PTEN: phosphatase and tensin homolog; Rab7: Ras-related GTPase 7; Raf: proto-oncogene tyrosine-protein kinase Raf; Ras: a GTPase; RIDD: regulated IRE-1-dependent decay; RIG-I: retinoic acid-inducible gene I; RIPK1/3: receptor-interacting protein kinase 1/3; RNF11/125: RING finger protein 11/125; ROS: reactive oxygen species; SHIP1: SH2-containing inositol 5' phosphatase 1; SOCS5: suppressor of cytokine signaling 5; Src: proto-oncogene tyrosine-protein kinase Src; ssRNA = single-stranded RNA; STAT: signal transducer and activator of transcription; TLR: toll-like receptor; TNFAIP3: tumor necrosis factor alpha-induced protein 3; TNFAR: tumor necrosis factor alpha receptor; TNF-α: tumor necrosis factor-alpha; TRAF6: tumor necrosis factor receptor-associated factor 6; TRIF: TIR-domain-containing adapter-inducing interferon-β; TRIM25: tripartite motif-containing 25; VCAM: vascular cell adhesion molecule; ZO-1: zonula occludens-1.
Keywords: Japanese encephalitis; glia; neuroinflammation; neuronal cell damage; therapy.
Conflict of interest statement
The authors report no conflict of interests.
Figures
Similar articles
-
Integrative RNA profiling of TBEV-infected neurons and astrocytes reveals potential pathogenic effectors.Comput Struct Biotechnol J. 2022 May 30;20:2759-2777. doi: 10.1016/j.csbj.2022.05.052. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35685361 Free PMC article.
-
Reprint of Neutrophil cell surface receptors and their intracellular signal transduction pathways.Int Immunopharmacol. 2013 Dec;17(4):1185-97. doi: 10.1016/j.intimp.2013.11.010. Epub 2013 Nov 18. Int Immunopharmacol. 2013. PMID: 24263067 Review.
-
Neutrophil cell surface receptors and their intracellular signal transduction pathways.Int Immunopharmacol. 2013 Nov;17(3):638-50. doi: 10.1016/j.intimp.2013.06.034. Epub 2013 Aug 30. Int Immunopharmacol. 2013. PMID: 23994464 Free PMC article. Review.
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
Cited by
-
The Infected Lungs and Brain Interface in COVID-19: The Impact on Cognitive Function.Neuroimmunomodulation. 2022;29(4):269-281. doi: 10.1159/000526653. Epub 2022 Nov 2. Neuroimmunomodulation. 2022. PMID: 36323239 Free PMC article. Review.
-
Peripheral nerve injury associated with JEV infection in high endemic regions, 2016-2020: a multicenter retrospective study in China.Emerg Microbes Infect. 2024 Dec;13(1):2337677. doi: 10.1080/22221751.2024.2337677. Epub 2024 Apr 22. Emerg Microbes Infect. 2024. PMID: 38578315 Free PMC article.
-
Monkeypox Virus Crosstalk with HIV: An Integrated Skin Transcriptome and Machine Learning Study.ACS Omega. 2023 Nov 29;8(49):47283-47294. doi: 10.1021/acsomega.3c07687. eCollection 2023 Dec 12. ACS Omega. 2023. PMID: 38107964 Free PMC article.
-
Intravenous AAV9 administration results in safe and widespread distribution of transgene in the brain of mini-pig.Front Cell Dev Biol. 2023 Jan 24;10:1115348. doi: 10.3389/fcell.2022.1115348. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36762127 Free PMC article.
-
Immune Functions of Astrocytes in Viral Neuroinfections.Int J Mol Sci. 2023 Feb 9;24(4):3514. doi: 10.3390/ijms24043514. Int J Mol Sci. 2023. PMID: 36834929 Free PMC article. Review.
References
-
- Turtle L, Solomon T. Japanese encephalitis - the prospects for new treatments. Nat Rev Neurol. 2018;14(5):298–313. - PubMed
-
- Mansfield KL, Hernandez-Triana LM, Banyard AC, et al. Japanese encephalitis virus infection, diagnosis and control in domestic animals. Vet Microbiol. 2017;201:85–92. - PubMed
-
- World Health Organization (WHO) . https://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis. Accessed 2019 May9.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
