Egr2 induction in spiny projection neurons of the ventrolateral striatum contributes to cocaine place preference in mice
- PMID: 33724178
- PMCID: PMC8057818
- DOI: 10.7554/eLife.65228
Egr2 induction in spiny projection neurons of the ventrolateral striatum contributes to cocaine place preference in mice
Abstract
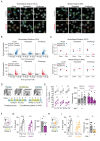
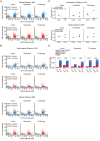
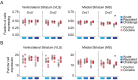
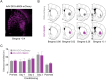
Drug addiction develops due to brain-wide plasticity within neuronal ensembles, mediated by dynamic gene expression. Though the most common approach to identify such ensembles relies on immediate early gene expression, little is known of how the activity of these genes is linked to modified behavior observed following repeated drug exposure. To address this gap, we present a broad-to-specific approach, beginning with a comprehensive investigation of brain-wide cocaine-driven gene expression, through the description of dynamic spatial patterns of gene induction in subregions of the striatum, and finally address functionality of region-specific gene induction in the development of cocaine preference. Our findings reveal differential cell-type specific dynamic transcriptional recruitment patterns within two subdomains of the dorsal striatum following repeated cocaine exposure. Furthermore, we demonstrate that induction of the IEG Egr2 in the ventrolateral striatum, as well as the cells within which it is expressed, are required for the development of cocaine seeking.
Keywords: IEG; cocaine; ensembles; mouse; neuroscience; reward; striatum; transcriptomics.
Plain language summary
The human brain is ever changing, constantly rewiring itself in response to new experiences, knowledge or information from the environment. Addictive drugs such as cocaine can hijack the genetic mechanisms responsible for this plasticity, creating dangerous, obsessive drug-seeking and consuming behaviors. Cocaine-induced plasticity is difficult to apprehend, however, as brain regions or even cell populations can react differently to the compound. For instance, sub-regions in the striatum – the brain area that responds to rewards and helps to plan movement – show distinct responses during progressive exposure to cocaine. And while researchers know that the drug immediately changes how neurons switch certain genes on and off, it is still unclear how these genetic modifications later affect behavior. Mukherjee, Gonzales et al. explored these questions at different scales, first focusing on how progressive cocaine exposure changed the way various gene programs were activated across the entire brain. This revealed that programs in the striatum were the most affected by the drug. Examining this region more closely showed that cocaine switches on genes in specific ‘spiny projection’ neuron populations, depending on where these cells are located and the drug history of the mouse. Finally, Mukherjee, Gonzales et al. used genetically modified mice to piece together cocaine exposure, genetic changes and modifications in behavior. These experiments revealed that the drive to seek cocaine depended on activation of the Egr2 gene in populations of spiny projection neurons in a specific sub-region of the striatum. The gene, which codes for a protein that regulates how genes are switched on and off, was itself strongly activated by cocaine intake. Cocaine addiction can have devastating consequences for individuals. Grasping how this drug alters the brain could pave the way for new treatments, while also providing information on the basic mechanisms underlying brain plasticity.
© 2021, Mukherjee et al.
Conflict of interest statement
DM, BG, RA, HT, MG, AC No competing interests declared
Figures





Similar articles
-
Subregion-specific rules govern the distribution of neuronal immediate-early gene induction.Proc Natl Acad Sci U S A. 2020 Sep 22;117(38):23304-23310. doi: 10.1073/pnas.1913658116. Epub 2019 Oct 21. Proc Natl Acad Sci U S A. 2020. PMID: 31636216 Free PMC article.
-
Stable immediate early gene expression patterns in medial prefrontal cortex and striatum after long-term cocaine self-administration.Addict Biol. 2017 Mar;22(2):354-368. doi: 10.1111/adb.12330. Epub 2015 Nov 24. Addict Biol. 2017. PMID: 26598422
-
Activity-Regulated Cytoskeleton-Associated Protein Accumulates in the Nucleus in Response to Cocaine and Acts as a Brake on Chromatin Remodeling and Long-Term Behavioral Alterations.Biol Psychiatry. 2017 Apr 1;81(7):573-584. doi: 10.1016/j.biopsych.2016.05.025. Epub 2016 Jun 16. Biol Psychiatry. 2017. PMID: 27567310
-
Multiple faces of BDNF in cocaine addiction.Behav Brain Res. 2015 Feb 15;279:240-54. doi: 10.1016/j.bbr.2014.11.018. Epub 2014 Nov 15. Behav Brain Res. 2015. PMID: 25449839 Free PMC article. Review.
-
Drug-activated cells: From immediate early genes to neuronal ensembles in addiction.Adv Pharmacol. 2021;90:173-216. doi: 10.1016/bs.apha.2020.09.006. Epub 2021 Feb 19. Adv Pharmacol. 2021. PMID: 33706932 Free PMC article. Review.
Cited by
-
Young transposable elements rewired gene regulatory networks in human and chimpanzee hippocampal intermediate progenitors.Development. 2022 Oct 1;149(19):dev200413. doi: 10.1242/dev.200413. Epub 2022 Oct 4. Development. 2022. PMID: 36052683 Free PMC article.
-
Luteolin Potentially Alleviates Methamphetamine Withdrawal-Induced Negative Emotions and Cognitive Deficits Through the AKT/FOXO1/HO-1 Signaling Pathway in the Prefrontal Cortex and Caudate Putamen.Int J Mol Sci. 2025 Jun 15;26(12):5739. doi: 10.3390/ijms26125739. Int J Mol Sci. 2025. PMID: 40565203 Free PMC article.
-
Psychedelic Targeting of Metabotropic Glutamate Receptor 2 and Its Implications for the Treatment of Alcoholism.Cells. 2023 Mar 22;12(6):963. doi: 10.3390/cells12060963. Cells. 2023. PMID: 36980303 Free PMC article. Review.
-
Environmental Enrichment Attenuates Repetitive Behavior and Alters the Functional Connectivity of Pain and Sensory Pathways in C58 Mice.Cells. 2024 Nov 21;13(23):1933. doi: 10.3390/cells13231933. Cells. 2024. PMID: 39682680 Free PMC article.
-
A Cocaine-Activated Ensemble Exerts Increased Control Over Behavior While Decreasing in Size.Biol Psychiatry. 2025 Mar 15;97(6):590-601. doi: 10.1016/j.biopsych.2024.06.008. Epub 2024 Jun 18. Biol Psychiatry. 2025. PMID: 38901723
References
-
- Amit I, Citri A, Shay T, Lu Y, Katz M, Zhang F, Tarcic G, Siwak D, Lahad J, Jacob-Hirsch J, Amariglio N, Vaisman N, Segal E, Rechavi G, Alon U, Mills GB, Domany E, Yarden Y. A module of negative feedback regulators defines growth factor signaling. Nature Genetics. 2007;39:503–512. doi: 10.1038/ng1987. - DOI - PubMed
-
- Atlan G, Terem A, Peretz-Rivlin N, Sehrawat K, Gonzales BJ, Pozner G, Tasaka GI, Goll Y, Refaeli R, Zviran O, Lim BK, Groysman M, Goshen I, Mizrahi A, Nelken I, Citri A. The claustrum supports resilience to distraction. Current Biology. 2018;28:2752–2762. doi: 10.1016/j.cub.2018.06.068. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

