The Long Non-Coding RNA HOTAIR Is a Critical Epigenetic Mediator of Angiogenesis in Diabetic Retinopathy
- PMID: 33724292
- PMCID: PMC7980040
- DOI: 10.1167/iovs.62.3.20
The Long Non-Coding RNA HOTAIR Is a Critical Epigenetic Mediator of Angiogenesis in Diabetic Retinopathy
Abstract
Purpose: Diabetic retinopathy (DR) remains a pressing issue worldwide. Abnormal angiogenesis is a distinct vascular lesion in DR, and research has established that vascular endothelial growth factor A (VEGF-A) is a primary mediator of such changes. However, limitations in current anti-VEGF therapies suggest that our understanding of molecular networks underlying ocular angiogenesis remains far from complete. Based on our long non-coding RNA (lncRNA) array analyses, HOX antisense intergenic RNA (HOTAIR) was identified as one of the top upregulated lncRNAs in high glucose-cultured human retinal endothelial cells (HRECs). Given the well-documented roles of HOTAIR in cancer, no studies have examined the epigenetic implications of HOTAIR in DR, and we investigated such relationships herein.
Methods: We used HRECs exposed to various glucose concentrations and epigenetic modulators to examine HOTAIR, angiogenic, and DR-related molecular markers. Oxidative stress, angiogenesis, and mitochondrial dysfunction were assessed. Retinal tissues of diabetic rodents and the vitreous humor and serum of patients with proliferative DR were also investigated.
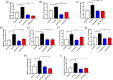
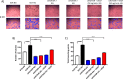
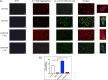
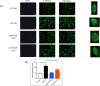
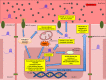
Results: Hyperglycemia significantly augmented HOTAIR expression in HRECs and promoted angiogenesis, oxidative damage, and mitochondrial aberrations. Similarly, vitreous humor and serum from proliferative DR patients and retinas from diabetic animals demonstrated increased HOTAIR expression compared to non-diabetic controls. HOTAIR knockdown protected against glucose-induced increases of angiogenic and diabetes-associated molecules in the retina. Mechanistically, we showed that HOTAIR exerts its capabilities by preventing oxidative stress and modulating epigenetic pathways involving histone methylation, histone acetylation, DNA methylation, and transcription factors.
Conclusions: Our findings suggest that HOTAIR is a critical lncRNA in the pathogenesis of DR and may potentially be important for diagnostic and therapeutic targeting.
Conflict of interest statement
Disclosure:
Figures



References
-
- Cho NH, Shaw JE, Karuranga S, et al. .. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018; 138: 271–281. - PubMed
-
- Biswas S, Chakrabarti S. Pathogenetic mechanisms in diabetic retinopathy: from molecules to cells to tissues. In: Kartha CC, Ramachandran S, Pillai RM, eds. Mechanisms of Vascular Defects in Diabetes Mellitus. Cham: Springer; 2017: 209–247.
-
- Grant MB, Afzal A, Spoerri P, Pan H, Shaw LC, Mames RN.. The role of growth factors in the pathogenesis of diabetic retinopathy. Expert Opin Investig Drugs. 2004; 13(10): 1275–1293. - PubMed
-
- Levy AP, Levy NS, Wegner S, Goldberg MA.. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995; 270(22): 13333–13340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

