Effectiveness of Standard Local Anesthetic Bupivacaine and Liposomal Bupivacaine for Postoperative Pain Control in Patients Undergoing Truncal Incisions: A Randomized Clinical Trial
- PMID: 33724391
- PMCID: PMC7967071
- DOI: 10.1001/jamanetworkopen.2021.0753
Effectiveness of Standard Local Anesthetic Bupivacaine and Liposomal Bupivacaine for Postoperative Pain Control in Patients Undergoing Truncal Incisions: A Randomized Clinical Trial
Abstract
Importance: Liposomal bupivacaine for pain relief is purported to last 3 days compared with 8 hours with standard bupivacaine. However, its effectiveness is unknown in truncal incisions for cardiothoracic or vascular operations.
Objective: To compare the effectiveness of single-administration standard bupivacaine vs liposomal bupivacaine in patients undergoing truncal incisions.
Design, setting, and participants: This randomized clinical trial enrolled patients undergoing sternotomy, thoracotomy, minithoracotomy, and laparotomy from a single cardiovascular surgery department in an academic medical center between November 2012 and June 2018. The study was powered to detect a Cohen effect size of 0.35 with a power of greater than 80%. Data analysis was performed from July to December 2018.
Intervention: Patients were randomized to standard bupivacaine or liposomal bupivacaine.
Main outcomes and measures: Pain was assessed over 3 postoperative days by the Numeric Rating Scale (NRS). Adjunctive opioids were converted to morphine equivalents units (MEU). NRS scores were compared using Wilcoxon rank-sum (3-day area under the curve) and 2-way nonparametric mixed models (daily scale score) to assess time-by-group interaction. Secondary outcomes included cumulative opioid consumption.
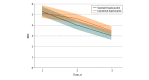
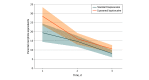
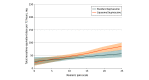
Results: A total of 280 patients were analyzed, with 140 in each group (single-administration standard bupivacaine vs liposomal bupivacaine). Mean (SD) age was 60.2 (14.4) years, and 101 of 280 patients (36%) were women. Irrespective of treatment assignment, pain decreased by a mean of approximately 1 point per day over 3 days (β = -0.87; SE = 0.11; mixed model regression P < .001). Incision type was associated with pain with patients undergoing thoracotomy (including minithoracotomy) reporting highest median (interquartile range [IQR]) pain scores on postoperative days 1 (liposomal vs standard bupivacaine, 6 [4-8] vs 5 [3-7]; P = .049, Wilcoxon rank-sum) and 2 (liposomal vs standard bupivacaine, 5 [4-7] vs 4 [2-6]; P = .003, Wilcoxon rank-sum) but not day 3 (liposomal vs standard bupivacaine, 3 [2-6] vs 3 [1-5]; P = .10, Wilcoxon rank-sum), irrespective of treatment group. Median (IQR) 3-day cumulative NRS was 12.0 (8.0-16.5) for bupivacaine and 13.5 (9.0-17.0) for liposomal bupivacaine (P = .15, Wilcoxon rank-sum) Furthermore, use of opioids was greater following liposomal bupivacaine compared with standard bupivacaine (median [IQR], 41.5 [21.3-73.8] MEU vs 33.0 [17.8-62.5] MEU; P = .03, Wilcoxon rank-sum). On multivariable analysis, no interaction by incision type was observed for mean pain scores or opioid use.
Conclusions and relevance: In this randomized clinical trial involving truncal incisions for cardiovascular procedures, liposomal bupivacaine did not provide improved pain control and did not reduce adjunctive opioid use compared with conventional bupivacaine formulation over 3 postoperative days.
Trial registration: ClinicalTrials.gov Identifier: NCT02111746.
Conflict of interest statement
Figures

References
-
- American Society of Anesthesiologists Task Force on Acute Pain Management . Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273. doi:10.1097/ALN.0b013e31823c1030 - DOI - PubMed
-
- Chou R, Gordon DB, de Leon-Casasola OA, et al. . Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157 [Erratum in J Pain. 2016;17(4)508-510]. doi:10.1016/j.jpain.2015.12.008 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

