Thoracic imaging tests for the diagnosis of COVID-19
- PMID: 33724443
- PMCID: PMC8078565
- DOI: 10.1002/14651858.CD013639.pub4
Thoracic imaging tests for the diagnosis of COVID-19
Update in
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2022 May 16;5(5):CD013639. doi: 10.1002/14651858.CD013639.pub5. Cochrane Database Syst Rev. 2022. PMID: 35575286 Free PMC article.
Abstract
Background: The respiratory illness caused by SARS-CoV-2 infection continues to present diagnostic challenges. Our 2020 edition of this review showed thoracic (chest) imaging to be sensitive and moderately specific in the diagnosis of coronavirus disease 2019 (COVID-19). In this update, we include new relevant studies, and have removed studies with case-control designs, and those not intended to be diagnostic test accuracy studies.
Objectives: To evaluate the diagnostic accuracy of thoracic imaging (computed tomography (CT), X-ray and ultrasound) in people with suspected COVID-19.
Search methods: We searched the COVID-19 Living Evidence Database from the University of Bern, the Cochrane COVID-19 Study Register, The Stephen B. Thacker CDC Library, and repositories of COVID-19 publications through to 30 September 2020. We did not apply any language restrictions.
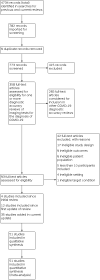
Selection criteria: We included studies of all designs, except for case-control, that recruited participants of any age group suspected to have COVID-19 and that reported estimates of test accuracy or provided data from which we could compute estimates.
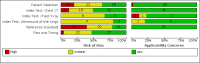
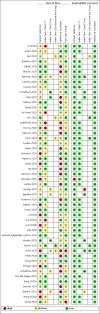
Data collection and analysis: The review authors independently and in duplicate screened articles, extracted data and assessed risk of bias and applicability concerns using the QUADAS-2 domain-list. We presented the results of estimated sensitivity and specificity using paired forest plots, and we summarised pooled estimates in tables. We used a bivariate meta-analysis model where appropriate. We presented the uncertainty of accuracy estimates using 95% confidence intervals (CIs).
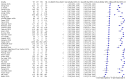
Main results: We included 51 studies with 19,775 participants suspected of having COVID-19, of whom 10,155 (51%) had a final diagnosis of COVID-19. Forty-seven studies evaluated one imaging modality each, and four studies evaluated two imaging modalities each. All studies used RT-PCR as the reference standard for the diagnosis of COVID-19, with 47 studies using only RT-PCR and four studies using a combination of RT-PCR and other criteria (such as clinical signs, imaging tests, positive contacts, and follow-up phone calls) as the reference standard. Studies were conducted in Europe (33), Asia (13), North America (3) and South America (2); including only adults (26), all ages (21), children only (1), adults over 70 years (1), and unclear (2); in inpatients (2), outpatients (32), and setting unclear (17). Risk of bias was high or unclear in thirty-two (63%) studies with respect to participant selection, 40 (78%) studies with respect to reference standard, 30 (59%) studies with respect to index test, and 24 (47%) studies with respect to participant flow. For chest CT (41 studies, 16,133 participants, 8110 (50%) cases), the sensitivity ranged from 56.3% to 100%, and specificity ranged from 25.4% to 97.4%. The pooled sensitivity of chest CT was 87.9% (95% CI 84.6 to 90.6) and the pooled specificity was 80.0% (95% CI 74.9 to 84.3). There was no statistical evidence indicating that reference standard conduct and definition for index test positivity were sources of heterogeneity for CT studies. Nine chest CT studies (2807 participants, 1139 (41%) cases) used the COVID-19 Reporting and Data System (CO-RADS) scoring system, which has five thresholds to define index test positivity. At a CO-RADS threshold of 5 (7 studies), the sensitivity ranged from 41.5% to 77.9% and the pooled sensitivity was 67.0% (95% CI 56.4 to 76.2); the specificity ranged from 83.5% to 96.2%; and the pooled specificity was 91.3% (95% CI 87.6 to 94.0). At a CO-RADS threshold of 4 (7 studies), the sensitivity ranged from 56.3% to 92.9% and the pooled sensitivity was 83.5% (95% CI 74.4 to 89.7); the specificity ranged from 77.2% to 90.4% and the pooled specificity was 83.6% (95% CI 80.5 to 86.4). For chest X-ray (9 studies, 3694 participants, 2111 (57%) cases) the sensitivity ranged from 51.9% to 94.4% and specificity ranged from 40.4% to 88.9%. The pooled sensitivity of chest X-ray was 80.6% (95% CI 69.1 to 88.6) and the pooled specificity was 71.5% (95% CI 59.8 to 80.8). For ultrasound of the lungs (5 studies, 446 participants, 211 (47%) cases) the sensitivity ranged from 68.2% to 96.8% and specificity ranged from 21.3% to 78.9%. The pooled sensitivity of ultrasound was 86.4% (95% CI 72.7 to 93.9) and the pooled specificity was 54.6% (95% CI 35.3 to 72.6). Based on an indirect comparison using all included studies, chest CT had a higher specificity than ultrasound. For indirect comparisons of chest CT and chest X-ray, or chest X-ray and ultrasound, the data did not show differences in specificity or sensitivity.
Authors' conclusions: Our findings indicate that chest CT is sensitive and moderately specific for the diagnosis of COVID-19. Chest X-ray is moderately sensitive and moderately specific for the diagnosis of COVID-19. Ultrasound is sensitive but not specific for the diagnosis of COVID-19. Thus, chest CT and ultrasound may have more utility for excluding COVID-19 than for differentiating SARS-CoV-2 infection from other causes of respiratory illness. Future diagnostic accuracy studies should pre-define positive imaging findings, include direct comparisons of the various modalities of interest in the same participant population, and implement improved reporting practices.
Copyright © 2021 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
Nayaar Islam has no known conflicts of interest.
Sanam Ebrahimzadeh has no known conflicts of interest.
Jean‐Paul Salameh has no known conflicts of interest.
Sakib Kazi has no known conflicts of interest.
Nicholas Fabiano has no known conflicts of interest.
Lee Treanor has no known conflicts of interest.
Marissa Absi has no known conflicts of interest.
Zachary Hallgrimson has no known conflicts of interest.
Mariska MG Leeflang has no known conflicts of interest.
Lotty Hooft has no known conflicts of interest.
Christian B van der Pol has no known conflicts of interest.
Ross Prager has no known conflicts of interest.
Samanjit Singh Hare has no known conflicts of interest.
Carole Dennie has no known conflicts of interest.
René Spijker: the Dutch Cochrane Centre (DCC) has received grants for performing commissioned systematic reviews. In no situation did the commissioner have any influence on the results of the work.
Jonathan J Deeks has no known conflicts of interest.
Jacqueline Dinnes has no known conflicts of interest.
Kevin Jenniskens has no known conflicts of interest.
Daniel Korevaar has no known conflicts of interest.
Jérémie F Cohen has no known conflicts of interest.
Ann Van den Bruel has no known conflicts of interest.
Yemisi Takwoingi has no known conflicts of interest.
Janneke van de Wijgert has no known conflicts of interest.
Johanna AAG Damen has no known conflicts of interest.
Junfeng Wang received a consultancy fee from Biomind, an Artificial Intelligence (AI) company providing machine intelligence solutions in medical imaging. The consultancy service was about design of clinical studies, not related to this review. The company had no influence on the results of the work.
Matthew McInnes has no known conflicts of interest.
Figures



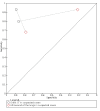
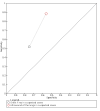
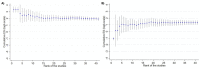
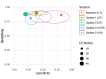
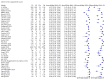


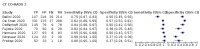

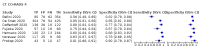
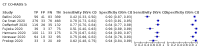
Update of
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Mar 16;3:CD013639. doi: 10.1002/14651858.CD013639.pub4. PMID: 33242342 Updated.
References
References to studies included in this review
Ai 2020a {published data only}
Aslan 2020 {published data only}
Bar 2020 {published data only}
Barbosa 2020 {published data only}
Bellini 2020 {published data only}
Besutti 2020 {published data only}
Borakati 2020 {published data only}
-
- Borakati A, Perera A, Johnson J, Sood T. Chest X-ray has poor diagnostic accuracy and prognostic significance in COVID-19: a propensity matched database study. medRxiv [Preprint] 2020. [DOI: DOI: 10.1101/2020.07.07.20147934] - PubMed
Cartocci 2020 {published data only}
-
- Cartocci G, Colaiacomo MC, Lanciotti S, Andreoli C, De Cicco ML, Brachetti G, et al. Chest CT for early detection and management of coronavirus disease (COVID-19): a report of 314 patients admitted to Emergency Department with suspected pneumonia. Radiologia Medica 2020;125(10):931-42. - PMC - PubMed
Caruso 2020 {published data only}
Choudhury 2020 {published data only}
Cozzi 2020 {published data only}
Debray 2020 {published data only}
Deng 2020 {published data only}
-
- Deng Z, Zhang X, Li Y, Xu H, Gang Y, Wang H, et al. Value of chest CT screening in the early COVID-19 outbreak. Chinese Journal of Radiology 2020;5(54):430-4.
De Smet 2020 {published data only}
-
- Smet KD, Smet DD, Demedts I, Bouckaert B, Ryckaert T, Laridon E, et al. Diagnostic power of chest CT for COVID-19: to screen or not to screen. medRxiv [Preprint] 2020. [DOI: ]
Dini 2020 {published data only}
Dofferhoff 2020 {published data only}
-
- Dofferhoff AS, Swinkels A, Sprong T, Berk Y, Spanbroek M, Nabuurs-Franssen MH, et al. Diagnostic algorithm for COVID-19 at the ER. Nederlands Tijdschrift voor Geneeskunde 2020;164:D5042. - PubMed
Ducray 2020 {published data only}
-
- Ducray V, Vlachomitrou AS, Bouscambert-Duchamp M, Si-Mohamed S, Gouttard S, Mansuy A, et al. Chest CT for rapid triage of patients in multiple emergency departments during COVID-19 epidemic: experience report from a large French university hospital. European Radiology 2020;31(2):795-803. [DOI: 10.1007/s00330-020-07154-4] - DOI - PMC - PubMed
Falaschi 2020 {published data only}
Fonsi 2020 {published data only}
-
- Fonsi GB, Sapienza P, Brachini G, Andreoli C, De Cicco ML, Cirillo B, et al. Is lung ultrasound imaging a worthwhile procedure for severe acute respiratory syndrome coronavirus 2 pneumonia detection? Journal of Ultrasound in Medicine 2020. [DOI: 10.1002/jum.15487 ] - PubMed
Fujioka 2020 {published data only}
Gezer 2020 {published data only}
Giannitto 2020 {published data only}
Gietema 2020 {published data only}
Guillo 2020 {published data only}
He 2020 {published data only}
Hermans 2020 {published data only}
Hernigou 2020 {published data only}
-
- Hernigou J, Cornil F, Poignard A, El Bouchaibi S, Mani J, Naouri JF, et al. Thoracic computerised tomography scans in one hundred eighteen orthopaedic patients during the COVID-19 pandemic: identification of chest lesions; added values; help in managing patients; burden on the computerised tomography scan department. International Orthopaedics (SICOT) 2020;44(8):1571–80. - PMC - PubMed
Herpe 2020 {published data only}
Hwang 2020 {published data only}
Ippolito 2020 {published data only}
-
- Ippolito D, Pecorelli A, Maino C, Capodaglio C, Mariani I, Giandola T, et al. Diagnostic impact of bedside chest X-ray features of 2019 novel coronavirus in the routine admission at the emergency department: case series from Lombardy region. European Journal of Radiology 2020;129:109092. - PMC - PubMed
Korevaar 2020 {published data only}
Krdzalic 2020 {published data only}
Kuzan 2020 {published data only}
-
- Kuzan TY, Murzoglu Altıntoprak K, et al. A comparison of clinical, laboratory and chest CT findings of laboratory-confirmed and clinically diagnosed COVID-19 patients at first admission. Diagnostic and Interventional Radiology [Epub ahead of print] 2020 Sep 2. [DOI: 10.5152/dir.2020.20270] - DOI - PMC - PubMed
Li 2020a {published data only}
Luo 2020a {published data only}
Luo 2020b {published data only}
Mei 2020 {published data only}
Miranda Magalhães Santos 2020 {published data only}
-
- Miranda Magalhães Santos JM, Paula Alves Fonseca A, Pinheiro Zarattini Anastacio E, Formagio Minenelli F, Furtado de Albuquerque Cavalcanti C, Borges da Silva Teles G. Initial results of the use of a standardized diagnostic criteria for chest computed tomography findings in coronavirus disease 2019. Journal of Computer Assisted Tomography 2020;44(5):647-51. - PubMed
Murphy 2020 {published data only}
Narinx 2020 {published data only}
Pare 2020 {published data only}
Patel 2020 {published data only}
-
- Patel M, Chowdhury J, Zheng M, Abramian O, Verga S, Zhao H, et al. High Resolution CHEST CT(HRCT) evaluation in patients hospitalized with COVID-19 infection. medRxiv [Preprint] 2020. [DOI: ]
Peng 2020 {published data only}
-
- Peng D, Zhang J, Xu Y, Liu Z, Wu P. Clinical analysis and early differential diagnosis of suspected pediatric patients with 2019 novel coronavirus infection. medRxiv [Preprint] 2020. [DOI: ]
Prokop 2020 {published data only}
Schiaffino 2020 {published data only}
-
- Schiaffino S, Tritella S, Cozzi A, Carriero S, Blandi L, Ferraris L, et al. Diagnostic performance of chest X-Ray for COVID-19 pneumonia during the SARS-CoV-2 pandemic in Lombardy, Italy. Journal of Thoracic Imaging 2020;35(4):W105–6. - PubMed
Schulze‐hagen 2020 {published data only}
Song 2020a {published data only}
Steuwe 2020 {published data only}
-
- Steuwe A, Rademacher C, Valentin B, Köhler M-H, Appel E, Keitel V, et al. Dose-optimised chest computed tomography for diagnosis of coronavirus disease 2019 (COVID-19) - evaluation of image quality and diagnostic impact. Journal of Radiological Protection 2020;40(3):877-91. - PubMed
Stevens 2020 {published data only}
Wang 2020a {published data only}
-
- Wang T, Xiong Z, Zhou H, Luo W, Tang H, Liu J. Design, validation, and clinical practice of standardized imaging diagnostic report for COVID-19. Zhong Nan da Xue Xue Bao. Yi Xue Ban [Journal of Central South University. Medical Sciences] 2020;45(3):229-35. - PubMed
Xiong 2020 {published data only}
-
- Xiong Z, Fu L, Zhou H, Liu JK, Wang AM, Huang Y, et al. Construction and evaluation of a novel diagnosis pathway for 2019-Corona virus disease. Zhonghua Yi Xue za Zhi 2020;100(16):1223-9. - PubMed
References to studies excluded from this review
Ai 2020b {published data only}
-
- Ai J, Gong J, Xing L, He R, Tian F, Wang J, et al. Analysis of factors associated early diagnosis in coronavirus disease 2019 (COVID-19). medRxiv [Preprint] 2020. [DOI: ]
Ai 2020c {published data only}
-
- Ai J, Chen J, Wang Y, Liu X, Fan W, Qu G, et al. The cross-sectional study of hospitalized coronavirus disease 2019 patients in Xiangyang, Hubei province. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.02.19.20025023] - DOI
Arentz 2020 {published data only}
Bai 2020a {published data only}
Bai 2020b {published data only}
Chang 2020 {published data only}
Chen 2020a {published data only}
-
- Chen S, Wu JJ, Li MZ, Xu DZ, Zhu YZ, Wang HC, et al. Clinical features of 109 cases of novel coronavirus pneumonia. Chinese Journal of Infectious Diseases 2020;38(12):E015. [DOI: 10.3760 / cma.j.issn.1000-6680.2020.0015]
Chen 2020b {published data only}
Chen 2020c {published data only}
Cheng 2020 {published data only}
-
- Cheng Z, Lu Y, Cao Q, Qin L, Pan Z, Yan F, et al. Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. American Journal of Roentgenology 2020;215(1):121-6. [DOI: 10.2214/ajr.20.22959 10.2214/AJR.20.22959.] - PubMed
Çinkooğlu 2020 {published data only}
Colombi 2020 {published data only}
Dai 2020 {published data only}
Ding 2020 {published data only}
Dong 2020 {published data only}
-
- Dong J, Wu L, Jin Q, Chen J, He J. Chest CT scan of hospitalized patients with COVID-19: a case-control study. medRxiv [Preprint] 2020. [DOI: ]
Guan 2020 {published data only}
Hao 2020 {published data only}
Himoto 2020 {published data only}
Huang 2020 {published data only}
Liang 2020 {published data only}
-
- Liang Y, Liang J, Zhou Q, Li X, Lin F, Deng Z, et al. Prevalence and clinical features of 2019 novel coronavirus disease (COVID-19) in the Fever Clinic of a teaching hospital in Beijing: a single-center, retrospective study. medRxiv [Preprint] 2020. [DOI: ]
Lu 2020 {published data only}
Mao 2020 {published data only}
Miao 2020a {published data only}
-
- Miao C, Zhuang J, Jin M, Xiong H, Huang P, Zhao Q, et al. A comparative multi-centre study on the clinical and imaging features of confirmed and unconfirmed patients with COVID-19. medRxiv [Preprint] 2020. [DOI: ]
Miao 2020b {published data only}
Pakray 2020 {published data only}
Poggiali 2020 {published data only}
Pu 2020 {published data only}
Siegel 2020 {published data only}
Song 2020b {published data only}
Tavare 2020 {published data only}
Wang 2020b {published data only}
Wu 2020a {published data only}
-
- Wu J, Feng CL, Xian XY, Qiang J, Zhang J, Mao QX, et al. Novel coronavirus pneumonia (COVID-19) CT distribution and sign features. Zhonghua Jie He He Hu Xi za Zhi [Chinese Journal of Tuberculosis and Respiratory Diseases] 2020;43(4):321-6. [DOI: 10.3760/cma.j.cn112147-20200217-00106 10.3760/cma.j.cn112147-20200217-00106.] - PubMed
Wu 2020b {published data only}
-
- Wu Q, Xing Y, Shi L, Li W, Gao Y, Pan S, et al. Epidemiological and clinical characteristics of children with coronavirus disease 2019. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.03.19.20027078] - DOI
Wu 2020c {published data only}
Wu 2020d {published data only}
Xie 2020 {published data only}
Xu 2020a {published data only}
Xu 2020b {published data only}
Yang 2020a {published data only}
Yang 2020b {published data only}
Yuan 2020 {published data only}
Additional references
Bossuyt 2015
BSTI 2020
-
- British Society of Thoracic Imaging. COVID-19 BSTI Reporting Templates and Codes. bsti.org.uk/covid-19-resources/covid-19-bsti-reporting-templates/ 2020 (accessed 4 January 2020).
Butler‐Laporte 2021
-
- Butler-Laporte G, Lawandi A, Schiller I, Yao MC, Dendukuri N, McDonald E, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Internal Medicine 2021:E1-8. [DOI: 10.1001/jamainternmed.2020.8876] - DOI - PMC - PubMed
China National Health Comission 2020
-
- China National Health Comission. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th Edition). kjfy.meetingchina.org/msite/news/show/cn/3337.html 2020 (accessed 24 January 2020).
Chu 2006
Cohen 2016
Datta 2020
Deeks 2020
Dinnes 2020
Harbord 2009
-
- Harbord RM, Whiting P. metandi: meta-analysis of diagnostic accuracy using hierarchical logistic regression. Stata Journal 2009;9(2):211-29. [DOI: ]
Hong 2018
Irwig 1995
Kucirka 2020
Lau 1992
Li 2020b
Loeffelholz 2020
McGrath 2017
-
- McGrath TA, McInnes MD, Langer FW, Hong J, Korevaar DA, Bossuyt PM. Treatment of multiple test readers in diagnostic accuracy systematic reviews-meta-analyses of imaging studies. European Journal of Radiology 2017;93:59–64. [DOI: ] - PubMed
Moher 2009
Ng 2020
Pedersen 2020
-
- Pedersen, TL. Package ‘ggforce’. www.ggforce.data-imaginist.com 2020 (accessed 5 January 2020).
R Core Team 2021 [Computer program]
-
- R Foundation for Statistical Computing R: A language and environment for statistical computing. R Core Team. Vienna, Austria: R Foundation for Statistical Computing, 2020.
Reitsma 2005
Rubin 2020
-
- Rubin GD, Ryerson CJ, Haramati LB, Sverzellati N, Kanne JP, Raoof S, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society. Radiology 2020;296(1):172–80. [DOI: 10.1148/radiol.2020201365] - DOI - PMC - PubMed
Salameh 2020b
Salehi 2020
Shi 2020
Simpson 2020
-
- Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, et al. Radiological Society of North America expert consensus document on reporting chest CT findings related to COVID-19: endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiology: Cardiothoracic Imaging 2020;2(2):e200152. [DOI: 10.1148/ryct.2020200152] - DOI - PMC - PubMed
StataCorp 2019 [Computer program]
-
- Stata Statistical Software. StataCorp, Version Release 16. College Station, TX: StataCorp LLC, 2019.
Stegeman 2020
Struyf 2020
-
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID‐19 disease. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665] - DOI - PMC - PubMed
Thomas 2010 [Computer program]
-
- EPPI-Centre Software EPPI-Reviewer 4.0: software for research synthesis. Thomas J, Brunton J, Graziosi S. London: Social Science Research Unit, Institute of Education, University of London: EPPI-Centre Software, 2010.
WHO 2020
-
- World Health Organization (WHO). WHO COVID-19 Case definition. WHO/2019-nCoV/Surveillance_Case_Definition/2020.1 2020 (accessed 17 October 2020).
Wickham 2016
-
- Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag, 2016.
References to other published versions of this review
Islam 2020
McInnes 2020
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

