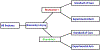
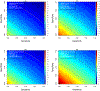
On the design and the analysis of stratified biomarker trials in the presence of measurement error
- PMID: 33724513
- PMCID: PMC8113124
- DOI: 10.1002/sim.8928
On the design and the analysis of stratified biomarker trials in the presence of measurement error
Abstract
A major emphasis in precision medicine is to optimally treat subgroups of patients who may benefit from certain therapeutic agents. And as such, enormous resources and innovative clinical trials designs in oncology are devoted to identifying predictive biomarkers. Predictive biomarkers are ones that will identify patients that are more likely to respond to specific therapies and they are usually discovered through retrospective analysis from large randomized phase II or phase III trials. One important design to consider is the stratified biomarker design, where patients will have their specimens obtained at baseline and the biomarker status will be assessed prior to random assignment. Regardless of their biomarker status, patients will be randomized to either an experimental arm or the standard of care arm. The stratified biomarker design can be used to test for a treatment-biomarker interaction in predicting a time-to event outcome. Many biomarkers, however, are derived from tissues from patients, and their levels may be heterogeneous. As a result, biomarker levels may be measured with error and this would have an adverse impact on the power of a stratified biomarker clinical trial. We present a trial design and an analysis framework for the stratified biomarker design. We show that the naïve test is biased and provide bias-corrected estimators for computing the sample size and the 95% confidence interval when testing for a treatment-biomarker interaction in predicting a time to event outcome. We propose a sample size formula that adjusts for misclassification and apply it in the design of a phase III clinical trial in renal cancer.
Keywords: biomarker; measurement error; precision medicine; randomized clinical trials; time-to-event endpoint.
© 2021 John Wiley & Sons, Ltd.
Figures
Similar articles
-
Bias in retrospective analyses of biomarker effect using data from an outcome-adaptive randomized trial.Clin Trials. 2019 Dec;16(6):599-609. doi: 10.1177/1740774519875969. Epub 2019 Oct 3. Clin Trials. 2019. PMID: 31581815
-
Randomized phase II trial designs with biomarkers.J Clin Oncol. 2012 Sep 10;30(26):3304-9. doi: 10.1200/JCO.2012.43.3946. Epub 2012 Aug 6. J Clin Oncol. 2012. PMID: 22869885 Free PMC article.
-
Quantitative evaluation of single-arm versus randomized phase II cancer clinical trials.Clin Trials. 2011 Jun;8(3):260-9. doi: 10.1177/1740774511401764. Epub 2011 Apr 20. Clin Trials. 2011. PMID: 21511687
-
Randomized phase II trials: a long-term investment with promising returns.J Natl Cancer Inst. 2011 Jul 20;103(14):1093-100. doi: 10.1093/jnci/djr218. Epub 2011 Jun 27. J Natl Cancer Inst. 2011. PMID: 21709274 Free PMC article. Review.
-
The FOCUS4 design for biomarker stratified trials.Chin Clin Oncol. 2015 Sep;4(3):35. doi: 10.3978/j.issn.2304-3865.2015.02.03. Chin Clin Oncol. 2015. PMID: 26408302 Review.
Cited by
-
Types and progress of clinical trial design for breast cancer: a narrative review.Transl Breast Cancer Res. 2023 Jul 30;4:20. doi: 10.21037/tbcr-23-22. eCollection 2023. Transl Breast Cancer Res. 2023. PMID: 38751463 Free PMC article. Review.
References
-
- Halabi S, Niedzwiecki D. Advancing precision oncology through biomarker-driven trials: Theory vs. practice. Chance. 2019; 32: 23–31. doi: 10.1080/09332480.2019.1695438 - DOI
-
- Herbst RS, Gandara DR, Hirsch FR, Redman MW, LeBlanc M, Mack PC. Lung master protocol LungMAP: A biomarker-driven protocol for accelerating development of therapies for squamous cell lung cancer: SWOG S1400. Clin Cancer Res. 2015; 21(7): 1514–1524. doi: 10.1158/1078-0432.CCR-13-3473 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials