Nanoparticle-Based Activatable Probes for Bioimaging
- PMID: 33724732
- PMCID: PMC7966733
- DOI: 10.1002/adbi.202000193
Nanoparticle-Based Activatable Probes for Bioimaging
Abstract
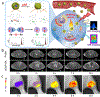
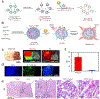
Molecular imaging can provide functional and molecular information at the cellular or subcellular level in vivo in a noninvasive manner. Activatable nanoprobes that can react to the surrounding physiological environment or biomarkers are appealing agents to improve the efficacy, specificity, and sensitivity of molecular imaging. The physiological parameters, including redox status, pH, presence of enzymes, and hypoxia, can be designed as the stimuli of the activatable probes. However, the success rate of imaging nanoprobes for clinical translation is low. Herein, the recent advances in nanoparticle-based activatable imaging probes are critically reviewed. In addition, the challenges for clinical translation of these nanoprobes are also discussed in this review.
Keywords: activatable nanoprobes; clinical translation; molecular imaging; nanomaterials; theranostics.
© 2021 Wiley-VCH GmbH.
Figures





References
-
- Herschman HR, Science 2003, 302, 605. - PubMed
-
- Weissleder R, Nature Reviews Cancer 2002, 2, 11. - PubMed
-
- Weissleder R, Mahmood U, Radiology 2001, 219, 316. - PubMed
-
- Liu C, Hou Y, Gao M, Advanced Materials 2014, 26, 6922. - PubMed
-
- Gao Z, Ma T, Zhao E, Docter D, Yang W, Stauber RH, Gao M, Small 2016, 12, 556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

