Enhancement of Wound Healing Efficacy by Increasing the Stability and Skin-Penetrating Property of bFGF Using 30Kc19α-Based Fusion Protein
- PMID: 33724733
- PMCID: PMC7996635
- DOI: 10.1002/adbi.202000176
Enhancement of Wound Healing Efficacy by Increasing the Stability and Skin-Penetrating Property of bFGF Using 30Kc19α-Based Fusion Protein
Abstract
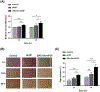
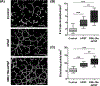
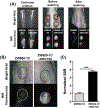
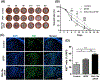
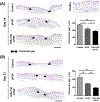
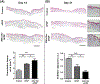
The instability of recombinant basic fibroblast growth factor (bFGF) is a major disadvantage for its therapeutic use and means frequent applications to cells or tissues are required for sustained effects. Originating from silkworm hemolymph, 30Kc19α is a cell-penetrating protein that also has protein stabilization properties. Herein, it is investigated whether fusing 30Kc19α to bFGF can enhance the stability and skin penetration properties of bFGF, which may consequently increase its therapeutic efficacy. The fusion of 30Kc19α to bFGF protein increases protein stability, as confirmed by ELISA. 30Kc19α-bFGF also retains the biological activity of bFGF as it facilitates the migration and proliferation of fibroblasts and angiogenesis of endothelial cells. It is discovered that 30Kc19α can improve the transdermal delivery of a small molecular fluorophore through the skin of hairless mice. Importantly, it increases the accumulation of bFGF and further facilitates its translocation into the skin through follicular routes. Finally, when applied to a skin wound model in vivo, 30Kc19α-bFGF penetrates the dermis layer effectively, which promotes cell proliferation, tissue granulation, angiogenesis, and tissue remodeling. Consequently, the findings suggest that 30Kc19α improves the therapeutic functionalities of bFGF, and would be useful as a protein stabilizer and/or a delivery vehicle in therapeutic applications.
Keywords: basic fibroblast growth factor; cell-penetrating proteins; protein stabilizer; skin wound healing; transdermal delivery.
© 2021 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of interest
The authors have declared that no competing interest exists.
Figures



References
-
- Kanazawa S, Fujiwara T, Matsuzaki S, Shingaki K, Taniguchi M, Miyata S, Tohyama M, Sakai Y, Yano K, Hosokawa K, PLoS One 2010, 5, e12228; - PMC - PubMed
- Song YH, Zhu YT, Ding J, Zhou FY, Xue JX, Jung JH, Li ZJ, Gao WY, Mol. Med. Rep 2016, 14, 3336; - PubMed
- O’keefe EJ, Chiu ML, Payne RE Jr, J. Investig. Dermatol 1988, 90; - PubMed
- Sogabe Y, Abe M, Yokoyama Y, Ishikawa O, Wound Repair Regen. 2006, 14, 457; - PubMed
- Przybylski M, J. Wound Care 2009, 18, 516. - PubMed
-
- Uchi H, Igarashi A, Urabe K, Koga T, Nakayama J, Kawamori R, Tamaki K, Hirakata H, Ohura T, Furue M, Eur. J. Dermatol 2009, 19, 461; - PubMed
- Nunes QM, Li Y, Sun C, Kinnunen TK, Fernig DG, PeerJ 2016, 4, e1535; - PMC - PubMed
- Akita S, Akino K, Imaizumi T, Hirano A, Wound Repair Regen. 2008, 16, 635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

