Access to Chiral Diamine Derivatives through Stereoselective Cu-Catalyzed Reductive Coupling of Imines and Allenamides
- PMID: 33724828
- PMCID: PMC8025098
- DOI: 10.1021/acs.joc.0c02971
Access to Chiral Diamine Derivatives through Stereoselective Cu-Catalyzed Reductive Coupling of Imines and Allenamides
Abstract
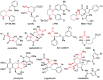
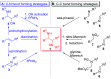
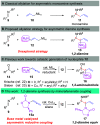
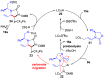
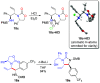
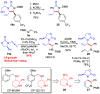
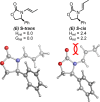
Chiral 1,2-diamino compounds are important building blocks in organic chemistry for biological applications and as asymmetric inducers in stereoselective synthesis that are challenging to prepare in a straightforward and stereoselective manner. Herein, we disclose a cost-effective and readily available Cu-catalyzed system for the reductive coupling of a chiral allenamide with N-alkyl substituted aldimines to access chiral 1,2-diamino synthons as single stereoisomers in high yields. The method shows broad reaction scope and high diastereoselectivity and can be easily scaled using standard Schlenk techniques. Mechanistic investigations by density functional theory calculations identified the mechanism and origin of stereoselectivity. In particular, the addition to the imine was shown to be reversible, which has implications toward development of catalyst-controlled stereoselective variants of the identified reductive coupling of imines and allenamides.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




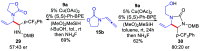
References
-
-
Reviews
- Lucet D.; Le Gall T.; Mioskowski C. The Chemistry of Vicinal Diamines. Angew. Chem., Int. Ed. 1998, 37, 2580–2627. 10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L. - DOI - PubMed
- Kotti S. R. S. S.; Timmons C.; Li G. Vicinal Diamino Functionalities as Privileged Structural Elements in Biologically Active Compounds and Exploitation of Their Synthetic Chemisty. Chem. Biol. Drug Des. 2006, 67, 101–114. 10.1111/j.1747-0285.2006.00347.x. - DOI - PubMed
- Viso A.; Fernandez de la Pradilla R.; Tortosa M.; Garcia A.; Flores A. Update 1 of: α,β-Diamino Acids: Biological Significance and Synthetic Approaches. Chem. Rev. 2011, 111, PR1–PR42. - PubMed
- Bergmeier S. C. The Synthesis of Vicinal Amino Alcohols. Tetrahedron 2000, 56, 2561–2576. 10.1016/S0040-4020(00)00149-6. - DOI
-
-
-
Selected examples:
- Desai M. C.; Lefkowitz S. L.; Thadeio P. F.; Longo K. P.; Snider R. M. Discovery of a Potent Substance P Antagonist: Recognition of the Key Molecular Determinant. J. Med. Chem. 1992, 35, 4911–4913. 10.1021/jm00104a018. - DOI - PubMed
- Farina V.; Brown J. D. Tamiflu: The Supply Problem. Angew. Chem., Int. Ed. 2006, 45, 7330–7334. 10.1002/anie.200602623. - DOI - PubMed
- Clark P. G. K.; Vieira L. C. C.; Tallant C.; Fedorov O.; Singleton D. C.; Rogers C. M.; Monteiro O.; Bennett J. M.; Baronio R.; Muller S.; Daniels D. L.; Mendez J.; Knapp S.; Brennan P. E.; Dixon D. J. LP99: Discovery and Synthesis of the First Selective BRD7/9 Bromodomain Inhibitor. Angew. Chem., Int. Ed. 2015, 54, 6217–6221. 10.1002/anie.201501394. - DOI - PMC - PubMed
- Curreli F.; Kwon Y. D.; Zhang H.; Scacalossi D.; Belov D. S.; Tikhonov A. A.; Andreev I. A.; Altieri A.; Kurkin A. V.; Kwong P. D.; Debnath A. K. Structure-Based Design of a Small Molecule CD4-Antagonis with Broad Spectrum Anti-HIV-1 Activity. J. Med. Chem. 2015, 58, 6909–6927. 10.1021/acs.jmedchem.5b00709. - DOI - PMC - PubMed
- Kamath A.; Ojima I. Advances in the Chemistry of b-Lactam and Its Medicinal Applications. Tetrahedron 2012, 68, 10640–10664. 10.1016/j.tet.2012.07.090. - DOI - PMC - PubMed
- D’Ambrosio M.; Guerriero A.; Debitus C.; Ribes O.; Pusset J.; Leroy S.; Pietra F. Agelastatin A, a New Skeleton Cytotoxic Alkaloid of the Oroidin Family. Isolation from the Axinellid Sponge Agelas dendromorpha of the Coral Sea. J. Chem. Soc., Chem. Commun. 1993, 1305–1306. 10.1039/c39930001305. - DOI
- Reichard G. A.; Stengone C.; Paliwal S.; Mergelsberg I.; Majmundar S.; Wang C.; Tiberi R.; McPhail A. T.; Piwinski J. J.; Shih B.-Y. Asymmetric Synthesis of 4,4-Disubstituted-2-Imidazoli-dinones: Potent NK1 Antagonists. Org. Lett. 2003, 5, 4249–4251. 10.1021/ol030104p. - DOI - PubMed
- De Clercq P. J. Biotin: A Timeless Challenge for Total Synthesis. Chem. Rev. 1997, 97, 1755–1792. 10.1021/cr950073e. - DOI - PubMed
- Welin E. R.; Ngamnithiporn A.; Klatte M.; Lapointe G.; Pototschnig G. M.; McDermott M. S. J.; Conklin D.; Gilmore C. D.; Tadross P. M.; Haley C. K.; Negoro K.; Glibstrup E.; Grunanger C. U.; Allan K. M.; Virgil S. C.; Slamon D. J.; Stoltz B. M. Concise Total Syntheses of (−)-Jorunnamycin A and (−)-Jorumycin Enabled by Asymmetric Catalysis. Science 2019, 363, 270–275. 10.1126/science.aav3421. - DOI - PMC - PubMed
- Iwatsuki M.; Nishihara-Tsukashima A.; Ishiyama A.; Namatame M.; Watanabe Y.; Handasah S.; Pranamuda H.; Marwoto B.; Matsumoto A.; Takahashi Y.; Otoguro K.; Omura S. Jogyamycin, a New Antiprotozoal Aminocyclopentitol Antibiotic, Produced by Streptomyces Sp. a-WM-JG-16.2. J. Antibiot. 2012, 65 (3), 169–171. 10.1038/ja.2011.136. - DOI - PubMed
- Nishimura S.; Matsunaga S.; Shibazaki M.; Suzuki K.; Furihata K.; van Soest R. W. M.; Fusetani N. Massadine, a Novel Geranylgeranyltransferase Type I Inhibitor from the Marine Sponge Stylissa aff. Org. Lett. 2003, 5, 2255–2257. 10.1021/ol034564u. - DOI - PubMed
-
-
- Doyle A. G.; Jacobsen E. N. Small-Molecule H-Bond Donors in Asymmetric Catalysis. Chem. Rev. 2007, 107, 5713–5743. 10.1021/cr068373r. - DOI - PubMed
- Taylor M. S.; Jacobsen E. N. Asymmetric Catalysis by Chiral Hydrogen-Bond Donors. Angew. Chem., Int. Ed. 2006, 45, 1520–1543. 10.1002/anie.200503132. - DOI - PubMed
-
- Bennani Y. L.; Hanessian S. trans-1,2-Diaminocyclohexane Derivatives as Chiral Reagents, Scaffolds, and Ligands for Catalysis: Applications in Asymmetric Synthesis and Molecular Recognition. Chem. Rev. 1997, 97, 3161–3196. 10.1021/cr9407577. - DOI - PubMed
- Trost B. M.; Machacek M. R.; Aponick A. Predicting Stereochemistry of Diphenylphosphino Benzoic Acid (DPPBA)-Based Palladium-Catalyzed Asymmetric Allylic Alkylation Reactions: a Working Model. Acc. Chem. Res. 2006, 39, 747–760. 10.1021/ar040063c. - DOI - PubMed
- Surry D. S.; Buchwald S. L. Diamine Ligands in Copper-Catalyzed Reactions. Chem. Sci. 2010, 1, 13–31. 10.1039/c0sc00107d. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

